Ceramide Metabolism: A Key Pathway in Lipid Signaling and Human Disease
Ceramide metabolism is a pivotal biochemical pathway at the crossroads of lipid signaling and cellular fate. Researchers have found that ceramides – a family of bioactive sphingolipids – serve not only as structural components of cell membranes but also as potent signaling molecules regulating processes like apoptosis (programmed cell death), inflammation, and insulin signaling. Dysregulation of ceramide metabolism has been linked to a host of clinical disorders: excess ceramide can trigger oxidative stress and inflammatory cascades, disrupt glucose and lipid homeostasis, and ultimately drive metabolic diseases such as type 2 diabetes and fatty liver disease. Given its far-reaching biological and clinical impact, ceramide metabolism is an important pathway for biologists and clinicians to study – understanding this pathway provides insight into how cells respond to stress and how metabolic disorders, neurodegeneration, and even cancers may be addressed by targeting lipid pathways.
Ceramide Metabolism Overview: Pathways and Regulation
Ceramides are simple sphingolipids consisting of a long-chain sphingoid base (typically sphingosine) with a fatty acid attached via an amide bond. In cells, ceramide sits at the core of the sphingolipid metabolic network, interconnecting multiple pathways of synthesis and breakdown. De novo ceramide biosynthesis begins in the endoplasmic reticulum (ER) and produces ceramide from basic precursors, whereas salvage pathways recycle complex sphingolipids (like sphingomyelin and glycosphingolipids) back into ceramide. Newly made ceramide in the ER is often transported to the Golgi apparatus (either by vesicles or a carrier protein called CERT) where it is converted into complex sphingolipids such as sphingomyelin and glucosylceramides. These complex sphingolipids integrate into cellular membranes (plasma membrane, organelles) and can be later broken down in lysosomes to regenerate ceramide, especially during cell stress or turnover.
The pathway is dynamically regulated: for example, conditions of cellular stress (heat shock, oxidative stress, chemotherapy) or inflammatory signals (tumor necrosis factor-α, Fas ligand) can rapidly activate ceramide production either via the de novo route or by sphingomyelinase enzymes that liberate ceramide from membrane sphingomyelin. Conversely, in normal physiology, ceramide levels are kept in check by conversion into less toxic metabolites (like sphingomyelin, glycosylceramides, or sphingosine-1-phosphate) to prevent unwarranted apoptosis. In essence, ceramide metabolism spans multiple cellular compartments – primarily the ER, Golgi, plasma membrane, and lysosomes – and serves as a hub that integrates nutrient status and stress signals with lipid-mediated responses.
Key Enzymes and Steps in Ceramide Biosynthesis and Breakdown
Ceramide metabolism involves a series of enzymatic reactions partitioned into distinct pathways (de novo synthesis, sphingomyelinase/salvage pathways, and catabolic routes). Below we outline the major reaction steps and enzymes, highlighting rate-limiting steps and regulatory nodes in each sub-pathway.
_1752137490_WNo_839d720.webp)
Sphingolipid metabolism pathway (Turpin-Nolan and Brüning, 2020)
De Novo Ceramide Synthesis (Endoplasmic Reticulum)
The de novo biosynthesis of ceramide is initiated in the cytosolic leaflet of the ER and starts with the condensation of serine and a fatty acyl-CoA (usually palmitoyl-CoA). This first step is catalyzed by serine palmitoyltransferase (SPT), a pivotal enzyme considered rate-limiting for sphingolipid production. SPT produces 3-ketodihydrosphingosine (also called 3-ketosphinganine), which is quickly reduced by 3-ketodihydrosphingosine reductase to form sphinganine (dihydrosphingosine). Next, ceramide synthases (CerS) – a family of six isoenzymes (CerS1–6) each preferring specific fatty acyl-CoA chain lengths – N-acylate sphinganine to generate dihydroceramide. This step determines the fatty acid chain composition of the resulting ceramide (for example, CerS1 adds C18:0 fatty acids, CerS2/CerS4 add very-long chains like C24, etc., contributing to diverse ceramide species). Finally, dihydroceramide desaturase (DEGS) introduces a trans-double bond at C4–C5 of dihydroceramide, yielding ceramide with the characteristic sphingosine base. The de novo pathway is a major source of ceramide in most cells and can be upregulated by metabolic oversupply – for instance, high levels of palmitate (from a high-fat diet) or serine can drive SPT to produce more ceramide. Notably, serine palmitoyltransferase is tightly regulated (as discussed later) because it controls the entry of substrates into sphingolipid biosynthesis.
Sphingomyelinase and Salvage Pathways (Lysosome/Plasma Membrane)
The salvage pathway refers to the recycling of complex sphingolipids back into ceramide. In lysosomes, sphingolipids undergo stepwise degradation by acid hydrolases: complex glycosphingolipids (e.g. gangliosides, glucosylceramides) are broken down by exoglycosidases to yield ceramide, and acid sphingomyelinase converts sphingomyelin into ceramide. The ceramide in lysosomes is then hydrolyzed by acid ceramidase, producing free sphingosine (the sphingoid base) and a free fatty acid. Notably, ceramide itself does not readily escape the lysosome, but sphingosine can exit to the cytosol. Once in the cytosol (or in the ER), sphingosine can be re-acylated by ceramide synthases to regenerate ceramide, effectively salvaging the sphingolipid backbones for reuse. This salvage pathway is continually active as cells turn over their membrane lipids, and it becomes especially important under conditions that stimulate sphingolipid degradation (for instance, during intense membrane trafficking or autophagy). A number of enzymes contribute to the salvage cycle – including sphingomyelinases, lysosomal β-glucosidase (glucocerebrosidase, which converts glucosylceramide to ceramide), various glycosidases, ceramidases, and the ceramide synthases for reassembly. Emerging evidence shows that ceramide produced via salvage pathways can significantly affect cell fate and signaling, sometimes explaining cases where blocking de novo synthesis did not eliminate ceramide accumulation.
Ceramide Catabolism and S1P Pathway
Ceramide can be further metabolized into other bioactive lipids, highlighting its central role as a hub. One important route is its breakdown to sphingosine-1-phosphate (S1P), a potent signaling molecule often with opposite effects to ceramide. This occurs in two steps: first, ceramide is cleaved by ceramidases (distinct isoforms exist for acid, neutral, and alkaline pH optima) to yield sphingosine. Then sphingosine kinases (SphK1 and SphK2) phosphorylate sphingosine to produce sphingosine-1-phosphate. S1P is exported out of cells by specific transporters and binds to S1P receptors on cell surfaces to elicit pro-survival, proliferative signals (for example, in the immune and vascular systems). S1P formation effectively removes sphingosine, tilting the balance away from ceramide – a concept known as the “sphingolipid rheostat,” in which high S1P promotes cell growth and migration while high ceramide (and sphingosine) promotes cell cycle arrest or apoptosis. S1P can be degraded by sphingosine-1-phosphate lyase, which irreversibly cleaves it into ethanolamine phosphate and a long-chain aldehyde, or dephosphorylated back to sphingosine by S1P phosphatases. These catabolic steps ensure that ceramide and S1P levels are finely tuned. In summary, the enzymes of ceramide metabolism form an intricate network: some build the ceramide pool, others convert or consume it, and together they maintain a balance between “pro-apoptotic” ceramides/sphingosine and “pro-survival” S1P in response to the cell’s needs.
 pathway - (1)_1752137760_WNo_700d700.webp)
Schematic representation of sphingosine-1-phosphate (S1P) pathway (Kreitzburg et al., 2018)
Biological Functions of Ceramides in Health and Disease
Ceramides are remarkably versatile molecules, serving not only as foundational components of cellular membranes but also as potent modulators of critical signaling pathways. Their influence spans structural integrity, cellular stress responses, and metabolic regulation.
Structural and Membrane Roles
Ceramides form the backbone of complex sphingolipids like sphingomyelin and glycosphingolipids, integral to plasma and organelle membranes. When generated, ceramide can cluster into rigid microdomains—so-called lipid rafts—that organize signaling molecules. This structural role directly enables receptor aggregation, as seen when ceramide-rich platforms facilitate death receptor clustering during apoptosis. Different tissues specialize in distinct ceramide species, underscoring the lipid’s tailored structural and signaling functions across cell types.
Mediators of Cell Stress and Apoptosis
As bioactive lipids, ceramides accumulate in response to stressors like chemotherapy or oxidative damage, triggering mitochondrial dysfunction and caspase activation. This positions ceramide as a pro-apoptotic signal within the “sphingolipid rheostat,” where high ceramide and sphingosine drive cell death, while sphingosine-1-phosphate (S1P) promotes survival. Thus, ceramide orchestrates cellular fate under stress, balancing proliferation versus apoptosis—a dynamic critical in cancer and immune responses.
Modulators of Metabolic Signaling
In metabolic tissues, ceramide disrupts insulin signaling by activating PP2A and PKCζ, blocking Akt and impairing glucose uptake. Elevated ceramide links nutrient overload, especially from saturated fats, to insulin resistance and hepatic steatosis. Ceramide metabolism also intersects with glycerolipid pathways: sphingomyelin synthesis from ceramide yields diacylglycerol, another key signaling lipid. Together, these pathways integrate lipid, amino acid, and energy metabolism, highlighting ceramide’s role as a central metabolic regulator.
Ceramide Metabolism in Disease, Drug Development, and Biotechnology
Ceramide metabolism has significant implications for human health and disease, as well as potential biotechnological applications. Dysregulation of this pathway is implicated in numerous diseases – ranging from metabolic syndromes to cancer and neurodegeneration – making enzymes of ceramide metabolism attractive targets for therapeutic intervention. Below, we highlight several key areas of clinical relevance and emerging applications.
Ceramide in Obesity, Diabetes, and Cardiometabolic Disease
Ceramide accumulation in tissues like liver, muscle, pancreas, and heart is a key factor linking obesity and type 2 diabetes (T2D) to insulin resistance. By activating PP2A and PKCζ, ceramides inhibit Akt, blocking glucose uptake and promoting hyperglycemia. Elevated circulating ceramides, especially species like C16:0 and C24:1, correlate with insulin resistance and predict T2D onset, emerging as superior biomarkers compared to LDL cholesterol. Beyond diabetes, high ceramide levels contribute to cardiovascular disease by driving endothelial dysfunction, inflammation, foam cell formation, and plaque instability, ultimately promoting atherosclerosis and heart failure. Mechanistically, ceramides induce mitochondrial damage and apoptosis in cardiac and vascular tissues. Encouragingly, lowering ceramide—through exercise, weight loss, or experimental drugs targeting SPT, ceramidase, or sphingosine kinase—improves insulin sensitivity and cardiac outcomes in models. Thus, ceramides act as “lipotoxic villains,” directly linking nutrient excess and inflammation to metabolic and cardiovascular deterioration, and represent promising targets for therapeutic intervention in cardiometabolic diseases.
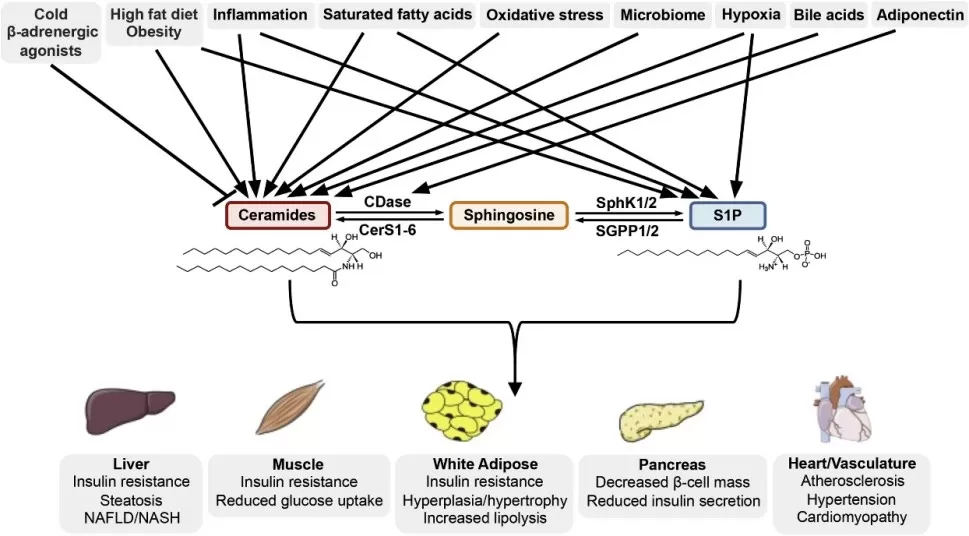
Ceramide and S1P are critical links in the development of metabolic disease (Green et al., 2021)
Ceramide Metabolism in Cancer
Ceramide metabolism is frequently altered in cancer, shaping both tumor survival and therapy response. While ceramides can trigger tumor cell apoptosis—mechanisms exploited by chemotherapy and radiation—many cancers upregulate enzymes like glucosylceramide synthase, sphingomyelin synthase, ceramide kinase, and sphingosine kinase to divert ceramide into pro-survival lipids like S1P. This metabolic rewiring lowers ceramide, elevates S1P, and fosters drug resistance. Studies show chemoresistant cancers often have reduced ceramide and increased ceramide-neutralizing activity. As a result, therapies targeting this balance are under development: SphK inhibitors (e.g., opaganib), GCS inhibitors (eliglustat), and ceramide-loaded nanoliposomes aim to raise ceramide or block its conversion, sensitizing tumors to treatment. Strategies like using safingol to inhibit SphK or fenretinide to accumulate dihydroceramides highlight efforts to tilt the “sphingolipid rheostat” toward apoptosis. Notably, higher ceramide/S1P ratios correlate with better outcomes in cancers like ovarian tumors. Thus, manipulating ceramide metabolism represents a promising avenue to overcome resistance and improve chemotherapy efficacy.
Ceramides in Neurodegeneration and Other Diseases
Ceramide metabolism plays pivotal roles in the nervous system. In Alzheimer’s and Parkinson’s disease, elevated brain ceramide levels promote amyloid-β generation, tau hyperphosphorylation, and neuronal apoptosis, contributing to plaque formation and synaptic loss. This has linked ceramide-driven “brain insulin resistance” to the concept of Alzheimer’s as “type 3 diabetes.” Additionally, ceramide pathways intersect with neuroinflammation, illustrated by sphingosine-1-phosphate receptor targeting in multiple sclerosis treatment (e.g., fingolimod). Ceramide dysregulation also underlies lysosomal storage disorders. Mutations in acid sphingomyelinase cause Niemann–Pick disease, leading to sphingomyelin and ceramide accumulation with neurodegeneration. Similarly, Gaucher’s and Fabry’s diseases involve glucosylceramide and globotriaosylceramide buildup. These genetic disorders have driven advances like enzyme replacement and substrate reduction therapies, including eliglustat, a GCS inhibitor reducing toxic glycosphingolipid storage. Together, these examples underscore ceramide’s broad impact on neurological health and disease.
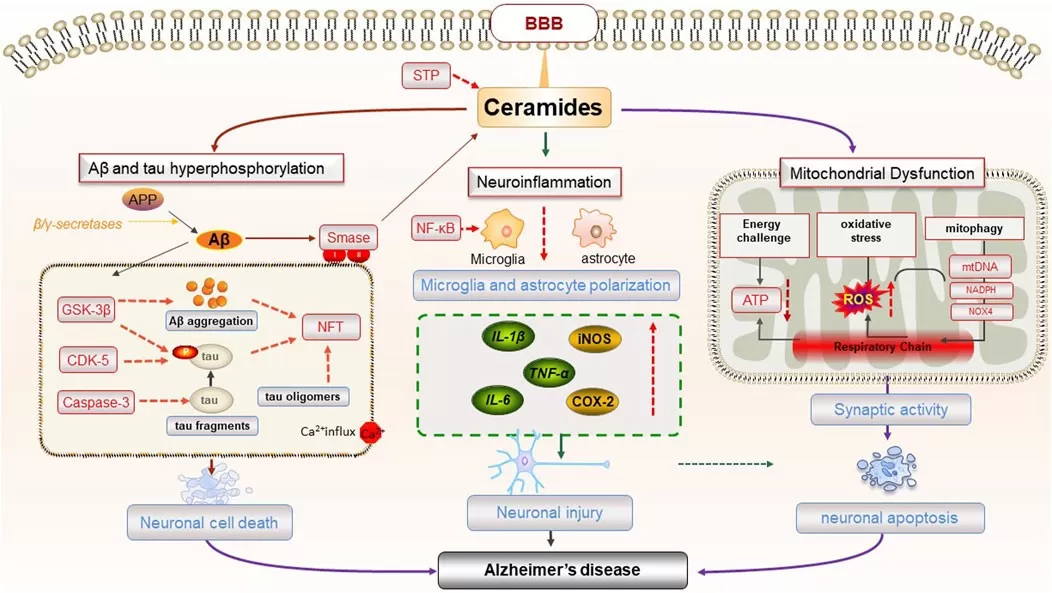
The pathogenesis of AD caused by ceramides (Pan et al., 2024)
Biotechnological and Industrial Applications of Ceramide Metabolism
On the biotechnological and industrial front, ceramides have garnered interest especially in dermatology and cosmetics. Ceramides are critical components of the skin’s stratum corneum barrier; topical ceramide supplements are used in creams to improve skin hydration and treat eczema. Traditionally, ceramides for cosmetics were extracted from animal sources or synthesized chemically, but nowadays fermentation technology offers a renewable source. Scientists have engineered yeast strains (like Saccharomyces cerevisiae) to produce human-type ceramides by introducing and tweaking sphingolipid biosynthetic genes. Yeast grows quickly and can be fermented at scale, making it an attractive “biofactory” for ceramide production. Indeed, some companies utilize yeast fermentation to mass-produce phytoceramides or sphingoid base precursors, which are then formulated into skincare products. Beyond cosmetics, metabolic engineering of microbes to produce specific ceramide species or analogues could facilitate drug development – for example, generating rare ceramide analogs for research or producing precursors to sphingosine-1-phosphate analog drugs. Another emerging application is in synthetic biology: researchers can modulate ceramide pathways in cell cultures to study apoptosis or to produce lipid metabolites on demand. All these applications underscore the versatility of ceramide metabolism not only as a subject of biomedical research but also as a target for innovation in therapeutics and biotechnology.
Analytical Approaches for Studying Ceramide Metabolism
Given the intricate roles of ceramide metabolism in health and disease, robust analytical strategies are essential to unravel its complexities. Modern techniques not only quantify ceramide species with remarkable precision but also illuminate their dynamic flux and regulatory networks. Below, we explore key methodologies that empower researchers to dissect ceramide pathways from molecular profiles to functional consequences.
Lipidomics and Mass Spectrometry Platforms
Modern lipidomics, built on LC–MS/MS, enables precise profiling of ceramides and related sphingolipids across tissues and biofluids. This technology separates ceramide species by chain length or saturation, detecting them at picomolar levels. Targeted LC–MS/MS assays can quantify dozens of ceramide variants in a single run, essential for biomarker studies linking ceramide ratios to disease or tracking pharmacodynamic effects of drugs. Untargeted methods (shotgun lipidomics) broaden discovery, revealing unexpected shifts in sphingolipid networks. GC–MS, though less used for intact ceramides, assists in analyzing sphingoid bases or fatty acids after hydrolysis. MS imaging adds spatial context by mapping ceramide gradients in tissue sections, invaluable for studying localized pathology in brain or tumors.
Stable Isotope Tracing and Flux Studies
To understand ceramide pathway dynamics, researchers use stable isotope tracing. By feeding cells labeled serine or palmitate (^13C/^2H), incorporation into ceramides is tracked over time via MS. Pulse-chase designs capture both synthesis and degradation rates. These experiments quantify flux through de novo versus salvage pathways, reveal how treatments alter ceramide turnover, and show rates of conversion to S1P or complex sphingolipids. Coupling data to computational models estimates enzyme activities indirectly, deepening insights into how metabolic stress or inhibitors reshape ceramide flow.
Multi-Omics and Genetic Dissection
Integrated studies combine lipidomics with transcriptomics or proteomics to uncover regulatory nodes. For example, upregulated ceramide synthase transcripts paired with lipid data highlight disease-driven enzyme shifts. CRISPR/Cas9 or siRNA knockdowns of enzymes like SPTLC2 causally link genes to ceramide phenotypes, validated by targeted sphingolipid quantification. Activity-based probes—fluorescent or clickable ceramide analogs—further uncover new ceramide-binding proteins, mapping out novel interactions within signaling networks.
Specialized Detection and Future Directions
Beyond MS, niche tools like ceramide-recognizing antibodies allow immunohistochemistry of lipid-rich domains, though lipids are challenging immunogens. High-resolution MS and ion mobility MS dissect structural ceramide isomers (e.g., C16:0 vs. C24:0), each potentially bearing distinct biological roles. Together, these cutting-edge analytical approaches decode ceramide complexity, supporting both fundamental research and the development of therapies targeting ceramide metabolism in disease contexts.
Advancing Ceramide Metabolism Research with MetwareBio’s Lipidomics Expertise
Understanding ceramide metabolism is not only academically fascinating – it has tangible implications for developing new interventions against metabolic disorders, cancer, and other diseases. As we have seen, this pathway interlinks with fundamental processes of cell survival and metabolism. MetwareBio, as a leader in proteomics, lipidomics, and metabolomics services, is ideally positioned to support and accelerate research in ceramide metabolism. We offer comprehensive lipidomics analysis platforms powered by state-of-the-art LC-MS/MS technology, enabling precise quantification of ceramides, sphingoid bases, and complex sphingolipids in a variety of sample types. By combining lipidomics with our metabolomics and bioinformatics services, we help researchers build a complete picture of how ceramide metabolism changes under different conditions or treatments. Whether you are investigating the role of ceramides in insulin resistance or screening for drugs that alter sphingolipid profiles in tumor cells, MetwareBio’s experienced team and advanced analytical platforms can provide the reliable data and insights you need. Together, we can decipher the complexities of ceramide metabolism and harness this knowledge for scientific breakthroughs and the development of novel therapeutic strategies. Unlock the potential of lipidomics with MetwareBio – and let ceramide metabolism reveal its secrets, from fundamental biology to clinical innovation.
Reference
1. Pan, Yun et al. “A review of the mechanisms of abnormal ceramide metabolism in type 2 diabetes mellitus, Alzheimer's disease, and their co-morbidities.” Frontiers in pharmacology vol. 15 1348410. 6 Feb. 2024, doi:10.3389/fphar.2024.1348410
2. Novgorodov, Sergei A, and Tatyana I Gudz. “Ceramide and mitochondria in ischemia/reperfusion.” Journal of cardiovascular pharmacology vol. 53,3 (2009): 198-208. doi:10.1097/FJC.0b013e31819b52d5
3. Turpin-Nolan, Sarah M, and Jens C Brüning. “The role of ceramides in metabolic disorders: when size and localization matters.” Nature reviews. Endocrinology vol. 16,4 (2020): 224-233. doi:10.1038/s41574-020-0320-5
4. Hussain, M Mahmood et al. “Mechanisms involved in cellular ceramide homeostasis.” Nutrition & metabolism vol. 9,1 71. 31 Jul. 2012, doi:10.1186/1743-7075-9-71
5. Sokolowska, Emilia, and Agnieszka Blachnio-Zabielska. “The Role of Ceramides in Insulin Resistance.” Frontiers in endocrinology vol. 10 577. 21 Aug. 2019, doi:10.3389/fendo.2019.00577
6. Kreitzburg, Kelly M et al. “Sphingolipid metabolism and drug resistance in ovarian cancer.” Cancer drug resistance (Alhambra, Calif.) vol. 1 (2018): 181-197. doi:10.20517/cdr.2018.06
7. Green, Christopher D et al. “Sphingolipids in metabolic disease: The good, the bad, and the unknown.” Cell metabolism vol. 33,7 (2021): 1293-1306. doi:10.1016/j.cmet.2021.06.006
8. Chaurasia, Bhagirath, and Scott A Summers. “Ceramides in Metabolism: Key Lipotoxic Players.” Annual review of physiology vol. 83 (2021): 303-330. doi:10.1146/annurev-physiol-031620-093815
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


