Glycoproteomics: Revolutionizing Clinical Testing and Diagnosis
In the world of modern medicine, diagnosing diseases early and accurately remains one of the greatest challenges. While traditional diagnostic tools such as imaging, blood tests, and genetic sequencing have provided invaluable insights, they often fall short in detecting diseases at their earliest stages or in tailoring treatments to individual patients. As we move towards more personalized healthcare, the need for more precise diagnostic techniques has never been greater.
This is where glycoproteomics comes into play. Glycoproteomics is the study of glycosylated proteins, which are critical markers for many biological processes and diseases. By analyzing the patterns of glycosylation—modifications that occur on proteins—researchers can uncover novel biomarkers for disease and develop better diagnostic tools. The potential of glycoproteomics in medicine is immense, as it promises to provide more accurate, earlier diagnoses, and even pave the way for more effective, personalized treatments.
In this article, we’ll explore how glycoproteomics is transforming clinical diagnostics, offering a glimpse into the future of medicine where early detection and individualized therapies are no longer a distant dream but an achievable reality.
Glycoproteomics: A Powerful New Tool
1. Glycosylation: The “Fingerprint” of Disease
Glycosylation, a process where sugar molecules are added to proteins, plays a crucial role in almost every biological process—from immune responses to cell signaling. These sugar modifications are not merely accessory molecules; they are functional players that govern the stability, structure, and activity of proteins. The patterns of glycosylation on proteins vary significantly across different tissues and even in different stages of disease, making them a powerful source of information for understanding biological processes.
In the context of disease, glycosylation patterns often undergo significant alterations. For example, in cancer, certain glycosylation changes can affect tumor growth, immune evasion, and metastasis. Similarly, in autoimmune disorders, the altered glycosylation of immune proteins can disrupt normal immune responses. As a result, these glycosylation changes act as a "fingerprint" of disease, making them valuable biomarkers for early diagnosis, disease monitoring, and therapeutic targeting. The ability to identify these changes could offer a much-needed advancement in diagnosing conditions that are difficult to detect with traditional methods, including many types of cancers and neurodegenerative diseases.
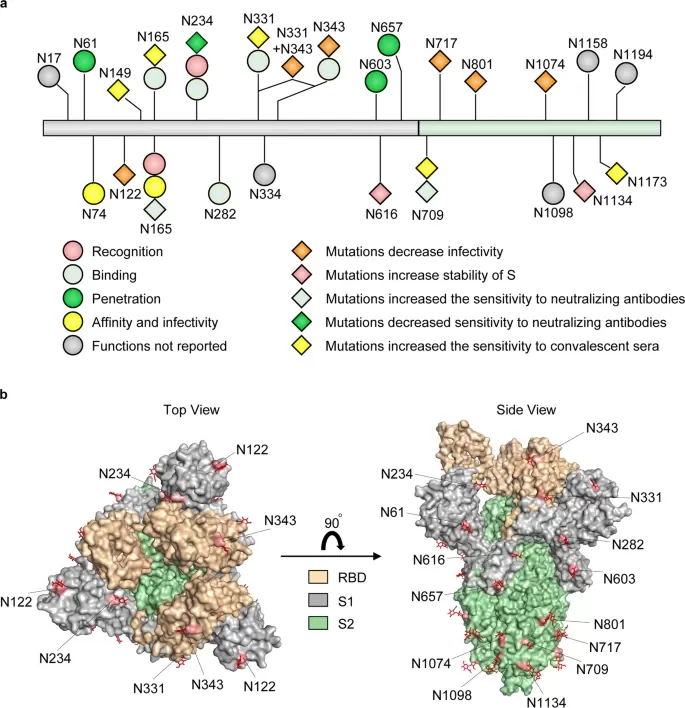
2. Glycoproteomics Techniques: Deciphering the Glycosylation Code
To fully unlock the potential of glycosylation as a biomarker, advanced analytical techniques are needed. Glycoproteomics is the field dedicated to the study of glycosylated proteins, and it relies heavily on cutting-edge technologies that can precisely identify and map the sugar modifications attached to proteins. Some of the most common techniques in glycoproteomics include:
High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS):
HPLC-MS is one of the most widely used methods in glycoproteomics. It separates glycoproteins based on their chemical properties and then analyzes their structure using mass spectrometry (MS). This allows researchers to identify glycosylation patterns with high sensitivity and specificity. By combining chromatography with mass spectrometry, this technique provides a powerful tool for analyzing complex glycoproteins in biological samples.
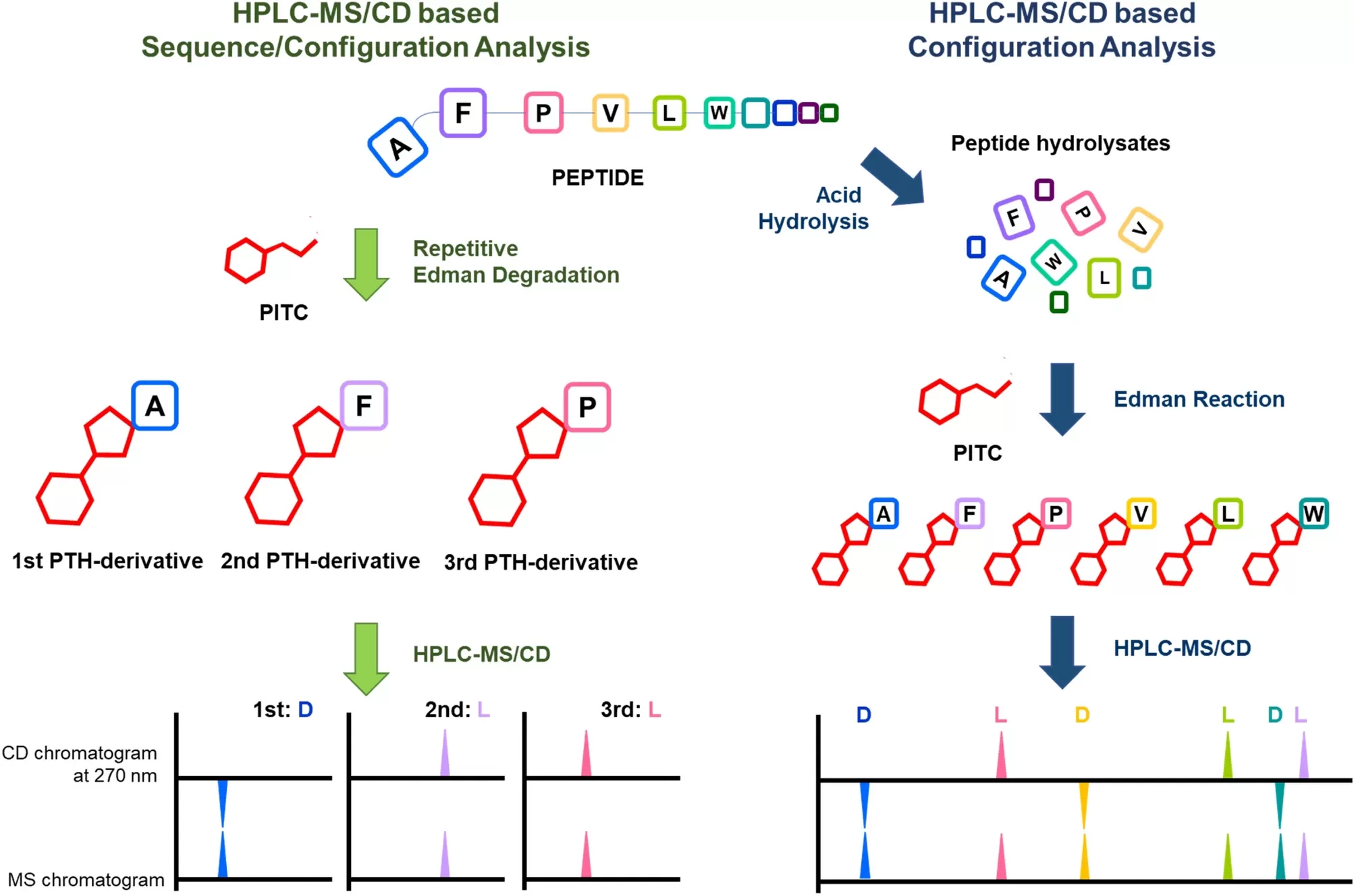
Capillary Electrophoresis-Mass Spectrometry (CE-MS):
Capillary electrophoresis (CE) separates glycoproteins based on their charge and size, and when paired with mass spectrometry, it can offer high-resolution analysis of glycosylation. This technique is particularly useful for analyzing small quantities of glycoproteins and can be applied to a wide range of clinical samples, including blood and urine.
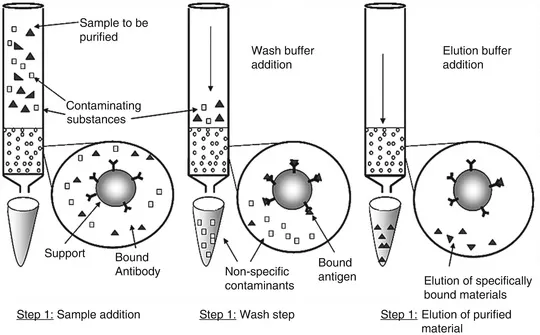
Immunoaffinity Chromatography:
Immunoaffinity chromatography is a method that uses antibodies to selectively capture glycoproteins from complex biological mixtures. This approach is highly specific and can be used to enrich glycoproteins that are of particular interest, allowing for more targeted analysis of disease-related glycosylation changes.
These techniques are essential for decoding the complex "glycosylation code" and are enabling researchers to uncover novel biomarkers that could revolutionize clinical diagnostics. By allowing for the precise identification of glycosylation patterns, these methods hold promise for improving early disease detection, monitoring progression, and developing more personalized therapeutic strategies.
Applications of Glycoproteomics in Clinical Testing
1. Cancer Diagnosis and Prognosis
Glycoproteomics has shown immense promise in revolutionizing the way we diagnose and monitor cancer. Traditional diagnostic tools, while effective in many cases, often fail to detect cancers at their earliest, most treatable stages. This is where glycoproteomics steps in. By analyzing the glycosylation patterns on proteins, researchers can identify specific biomarkers associated with various types of cancers, offering a much more sensitive and early detection method.
For instance, the glycosylation of specific proteins has been found to be altered in various cancers, including breast, prostate, and lung cancers. These changes can provide insights not only into the presence of cancer but also into the subtype of the tumor, helping to categorize tumors more accurately for personalized treatment. Additionally, glycoproteomics can be used to predict cancer prognosis by identifying biomarkers that correlate with tumor aggressiveness and patient outcomes. This approach has been successfully applied in several studies, including the identification of glycosylation changes in serum proteins as early indicators of cancer progression.
Moreover, glycoproteomics is proving valuable in monitoring treatment response. Since glycosylation changes are often linked to tumor biology, tracking these alterations during and after treatment can provide critical insights into how well a patient is responding to therapy. This can guide clinicians in adjusting treatment plans for optimal results, making cancer management more dynamic and personalized.
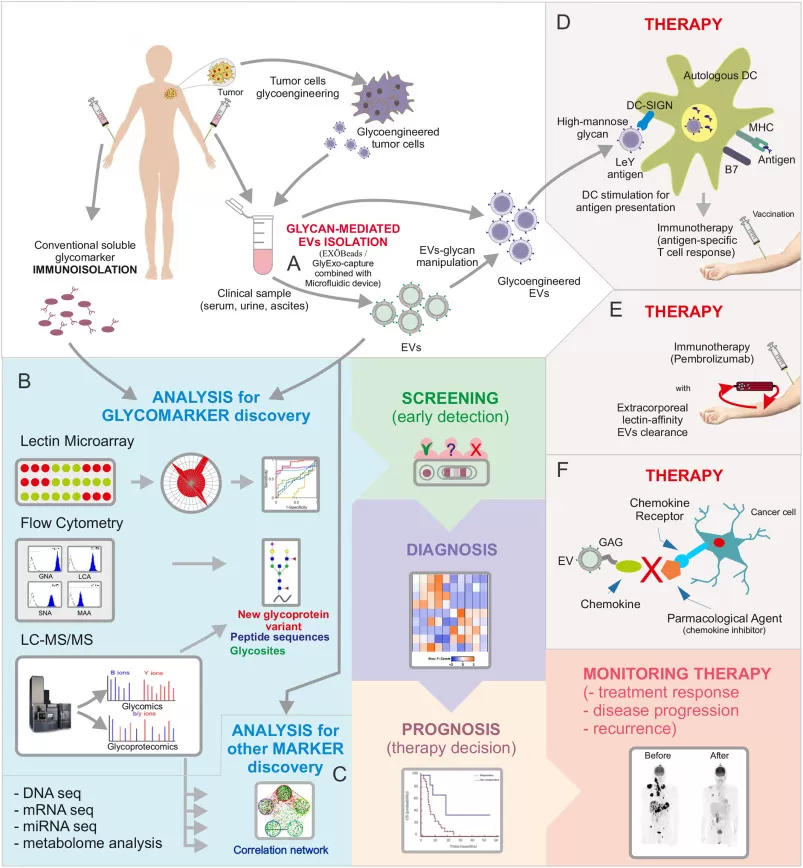
2. Diagnosis and Treatment of Other Diseases
Beyond cancer, glycoproteomics has significant potential in the diagnosis and treatment of a wide range of other diseases. In diabetes, for example, altered glycosylation patterns in proteins such as hemoglobin can provide early markers for disease onset and progression. These biomarkers can help identify individuals at risk of developing diabetes long before conventional symptoms appear, enabling earlier intervention and better disease management.
In cardiovascular diseases, glycosylation changes in proteins involved in blood clotting and inflammation can serve as indicators of heart disease risk, offering a way to predict heart attacks or strokes before they happen. Glycoproteomics also has a role in infectious diseases. Pathogens often modify host cell proteins during infection, and glycosylation patterns in these proteins can offer valuable diagnostic insights into the type of infection and its severity. By identifying these markers, clinicians can choose the most effective treatment plans early on.
In the realm of autoimmune diseases, glycoproteomics can provide information about the dysregulation of the immune system. Changes in the glycosylation of immune proteins can serve as indicators of inflammatory activity, helping doctors diagnose autoimmune diseases at an earlier stage, track their progression, and monitor how well patients are responding to treatments.
In all of these areas—diabetes, cardiovascular diseases, infections, and autoimmune disorders—glycoproteomics holds the potential to not only improve early diagnosis but also to pave the way for precision medicine. By analyzing specific glycosylation patterns, clinicians can tailor treatments more accurately to the individual patient, leading to more effective therapies and better outcomes. Additionally, tracking glycosylation changes over time can provide valuable insights into disease progression and treatment efficacy.
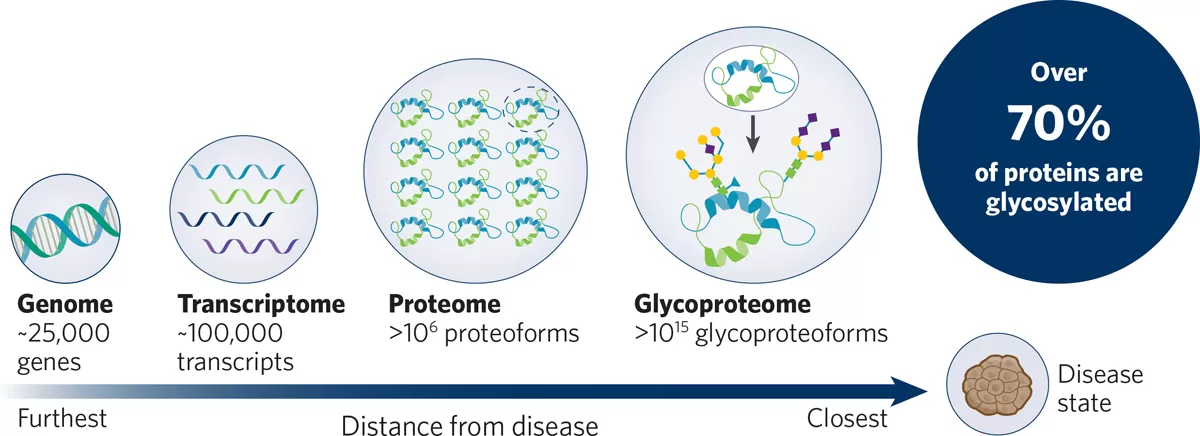
Future of Glycoproteomics
1. Challenges and Opportunities
As glycoproteomics continues to gain traction in the medical field, it faces a number of challenges that must be overcome to fully realize its potential in clinical diagnostics and personalized treatments. One of the primary challenges is the technical complexity involved in analyzing glycosylation patterns. The vast diversity of glycosylation modifications, coupled with the intricate nature of glycoproteins, makes their identification and analysis highly complex. Current technologies, while advanced, still struggle with achieving high throughput, sensitivity, and accuracy, especially when working with low-abundance glycoproteins in complex biological samples.
Another significant hurdle is the standardization of glycoproteomics techniques. Unlike genomics and proteomics, where there are established protocols and databases, the field of glycoproteomics lacks universal standards, making it difficult to compare results across different studies and laboratories. This issue complicates the integration of glycoproteomics into routine clinical practice, where reproducibility and reliability are paramount.
Additionally, the data analysis and interpretation of glycoproteomics data present significant challenges. The sheer volume and complexity of data generated require sophisticated computational tools and algorithms to decipher meaningful biological information. Developing accurate and efficient methods for data analysis remains a key focus for researchers and industry leaders.
Despite these challenges, the field of glycoproteomics also presents tremendous opportunities. Advances in technology, such as improvements in mass spectrometry, microfluidics, and bioinformatics tools, are gradually addressing these issues. Moreover, the establishment of comprehensive glycosylation databases and the development of more user-friendly analysis platforms hold promise for accelerating the application of glycoproteomics in clinical settings. As these technologies evolve, the gap between research and clinical practice is expected to narrow, unlocking new avenues for early diagnosis, personalized therapies, and disease monitoring.
2. Glycoproteomics: Driving Personalized Medicine
The future of glycoproteomics is inextricably linked to the field of personalized medicine. One of the most compelling advantages of glycoproteomics is its ability to capture the subtle and dynamic changes in glycosylation that reflect an individual’s unique biological state. This capability opens the door to precision diagnostics and targeted treatments that are tailored to the specific needs of each patient.
For example, glycoproteomics can be used to identify patient-specific glycosylation patterns that correlate with disease risk, progression, and treatment response. This would enable clinicians to make more informed decisions about the best therapeutic approach for each patient, leading to improved outcomes and fewer adverse effects. By understanding the glycosylation profile of a patient’s biomarkers, healthcare providers can optimize drug selection, dosage, and timing, ensuring that treatments are as effective as possible.
In the future, glycoproteomics may play a pivotal role in the development of novel drugs and therapeutic strategies. By identifying glycosylation-based biomarkers, pharmaceutical companies could develop drugs that specifically target these biomarkers, offering more effective treatments with fewer side effects. Furthermore, glycoproteomics could facilitate the early detection of diseases like cancer and autoimmune disorders, potentially leading to interventions before diseases reach advanced stages, thus improving prognosis and reducing healthcare costs.
Glycoproteomics also holds the potential to reduce healthcare costs in the long term. With more precise diagnostics and personalized treatments, patients could receive the right treatment at the right time, minimizing the need for trial-and-error approaches and preventing unnecessary hospitalizations or treatments. As glycoproteomics becomes a mainstream tool in clinical practice, it could shift the focus from reactive, one-size-fits-all medicine to proactive, individualized care, ultimately leading to more efficient healthcare systems.
Conclusion
Glycoproteomics is undoubtedly reshaping the landscape of clinical diagnostics and treatment. By analyzing the glycosylation patterns of proteins, this innovative field opens up new possibilities for early disease detection, personalized treatments, and monitoring disease progression. From cancer diagnostics to chronic disease management, the potential of glycoproteomics to improve patient outcomes is immense, offering a more precise, targeted, and effective approach to healthcare.
As we look toward the future, glycoproteomics will become an indispensable part of modern medicine. With ongoing advancements in technology and data analysis, the integration of glycoproteomics into clinical practice is set to unlock breakthroughs that were once thought to be out of reach. By providing deeper insights into the molecular mechanisms of disease, glycoproteomics promises to enhance not only diagnostic accuracy but also the development of more effective, individualized therapies.
The field of glycoproteomics is still evolving, and its potential is vast. Researchers, clinicians, and healthcare professionals alike should remain engaged with the ongoing developments in this exciting field. As we continue to explore the complexities of glycosylation and its role in human health, glycoproteomics will undoubtedly play a pivotal role in shaping the future of medicine, bringing new hope for better diagnostics, personalized treatments, and improved health outcomes worldwide.
Reference
1. Gong, Y., Qin, S., Dai, L. et al. The glycosylation in SARS-CoV-2 and its receptor ACE2. Sig Transduct Target Ther 6, 396 (2021). https://doi.org/10.1038/s41392-021-00809-8
2. Hahn, D., Wang, W., Choi, H. et al. Determination of sequence and absolute configuration of peptide amino acids by HPLC–MS/CD-based detection of liberated N-terminus phenylthiohydantoin amino acids. Sci Rep 12, 10285 (2022). https://doi.org/10.1038/s41598-022-14205-x
3. Frantzi, M., Gomez Gomez, E., Blanca Pedregosa, A. et al. CE–MS-based urinary biomarkers to distinguish non-significant from significant prostate cancer. Br J Cancer 120, 1120–1128 (2019). https://doi.org/10.1038/s41416-019-0472-z
4. Fitzgerald, J., Leonard, P., Darcy, E., O’Kennedy, R. (2011). Immunoaffinity Chromatography. In: Walls, D., Loughran, S. (eds) Protein Chromatography. Methods in Molecular Biology, vol 681. Humana Press. https://doi.org/10.1007/978-1-60761-913-0_3
5. Karolina Grzesik, Marcelina Janik, Dorota Hoja-Łukowicz, The hidden potential of glycomarkers: Glycosylation studies in the service of cancer diagnosis and treatment, Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, Volume 1878, Issue 3, 2023, 188889, ISSN 0304-419X, https://www.sciencedirect.com/science/article/pii/S0304419X23000380.
6. InterVenn Biosciences. Glycoproteomics: finding the new sweet spot for diagnosing and treating disease. Biopharma Dealmakers (Biopharm Deal), ISSN 2730-6283. https://www.nature.com/articles/d43747-022-00121-6
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


