Exploring Gut Mycobiome Dynamics in Pregnancy: Unveiling Connections to Metabolism and Health
Pregnancy is an important and unique stage in a mother’s life, as well as the beginning of life for the offspring, impacting the health and long-term disease outcomes of both mother and child. During pregnancy, various organs in the maternal digestive, endocrine, and immune systems undergo adaptive changes to support or accommodate the fetus’s growth and development in the womb. Additionally, the gut microbiota, widely recognized as a virtual "organ," also undergoes dynamic structural and functional changes during pregnancy. These changes may not merely be passive adaptations to the host's physiological changes but could actively influence the host's physiological activities. The human gut contains not only a large number of bacteria but also significant fungi. The interactions between gut bacteria and fungi, as important components of the gut microecology, are inevitable, including mutualism, commensalism, parasitism, or competition. Furthermore, the interactions between gut bacteria and fungi are closely related to the host's health status. However, previous studies on the gut microbiota in pregnant women have mainly focused on gut bacteria and their potential impact on pregnancy health, lacking an exploration of gut fungi during pregnancy and their impact on metabolic health or adverse pregnancy outcomes.
Recently, a research team published a new study in Gut titled "Landscape of the gut mycobiome dynamics during pregnancy and its relationship with host metabolism and pregnancy health (https://doi.org/10.1136/gutjnl-2024-332260)." This study, based on a large-scale cohort of pregnant women (4800 participants), integrated dietary surveys, clinical examinations, and data from the mycobiome, bacteriome, and metabolome. It comprehensively depicted the factors influencing the composition and structure of gut fungi and their dynamic changes during pregnancy. Additionally, it analyzed the associations and potential mechanisms between gut fungi, host metabolism, and pregnancy health, providing important insights into the impact of gut microorganisms on healthy pregnancy from the perspective of gut fungi.
Participant characteristics and gut mycobiome composition
This study was based on the Tongji-Huaxi-Shuangliu Birth Cohort (THSBC) and included 4800 participants with available ITS2 sequencing data, dietary information, and clinical records to comprehensively profile the gut mycobiome in pregnant women and investigate factors contributing to its variations. To examine the impact of pregnancy on the gut mycobiome and its associations with host metabolism, a subcohort of 1059 participants was established, included 514 women who gave birth to preterm (n=240), low birthweight (n=137), or macrosomia (n=216) infants, and 545 randomly selected controls without these adverse outcomes. ITS2 sequencing was performed on the entire cohort of 4800 participants, while shotgun metagenomics sequencing was done on first trimester samples from the subcohort (n=1059). Additionally, within the subcohort, 750 participants had ITS2 sequencing and 748 had 16S sequencing data across all trimesters.
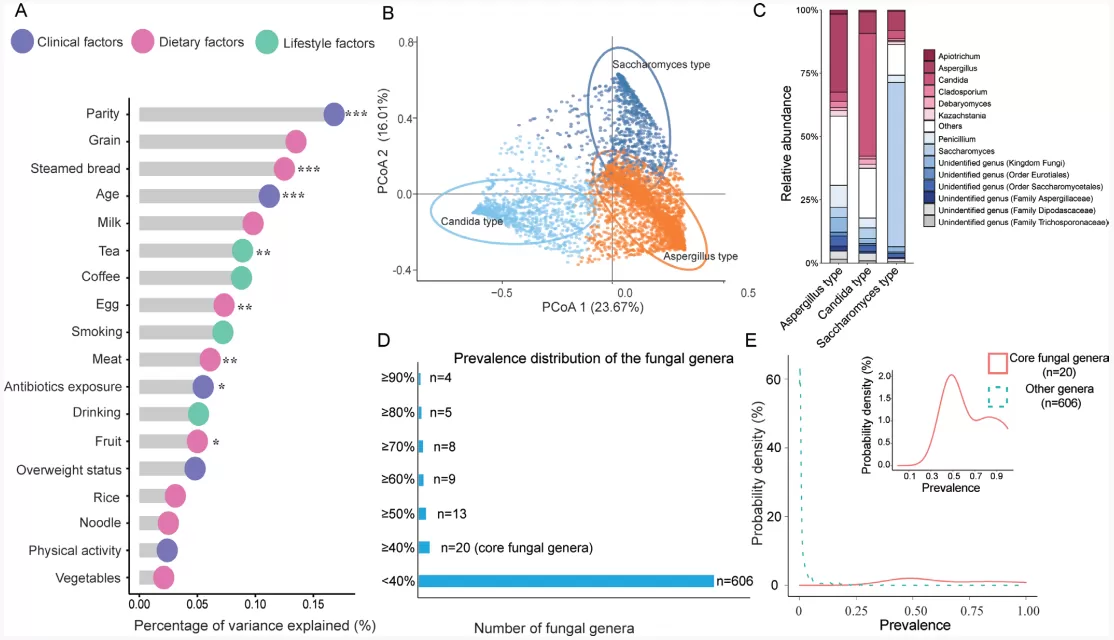
The study found that age and parity are major factors influencing the composition of the gut mycobiome, with antibiotic use and dietary factors also having significant impacts. We observed three primary fungal enterotypes: Saccharomyces-dominated (26.5%), Candida-dominated (18.8%), and Aspergillus-dominated (54.7%). Women who are primiparous and prefer consuming steamed bread are more likely to have a Saccharomyces-dominated enterotype, while women with a habit of tea consumption are more likely to have a Candida-dominated enterotype. In the samples of 4800 participants, a total of 626 fungal genera were identified, with over 96% of them having a detection rate of less than 40%. Among these, 20 fungal genera with a detection rate exceeding 40% were considered core fungal genera for further analysis.
Compositional dynamics of the gut mycobiome during pregnancy in the longitudinal subcohort
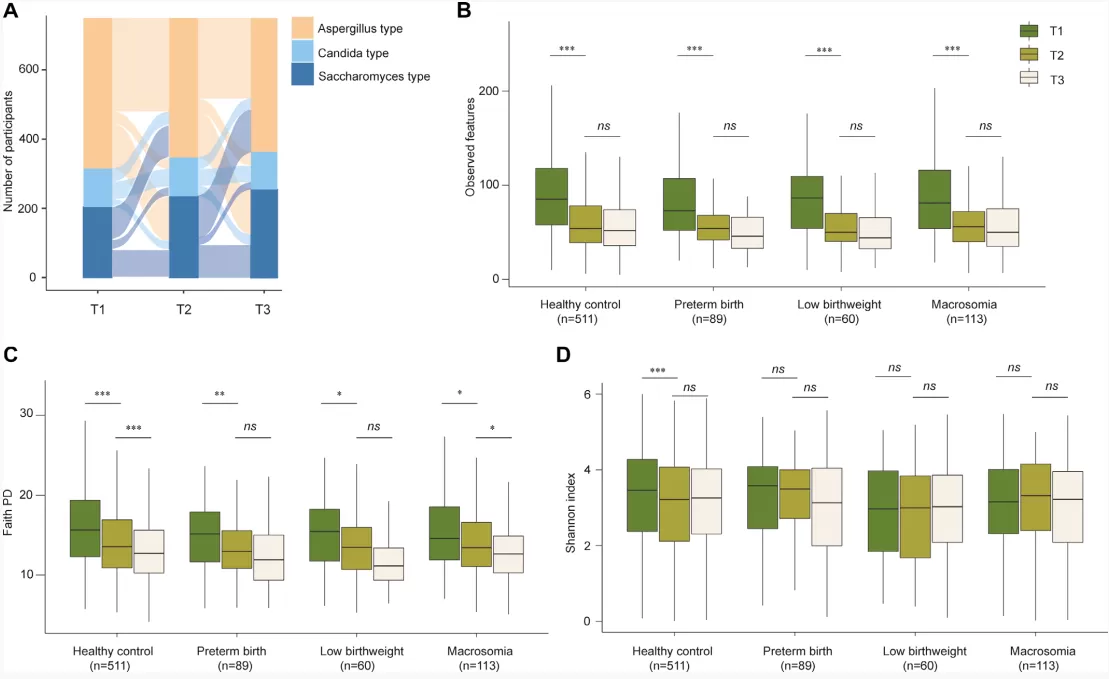
Via the analysis of the gut mycobiome of 750 pregnant women in the longitudinal subcohort, the study found significant compositional changes from the first trimester (T1) to the second trimester (T2), but minimal changes from T2 to the third trimester (T3). Up to 68.5% of the participants experienced shifts in their fungal enterotypes during pregnancy. The proportion of Saccharomyces-dominated enterotypes increased, the proportion of Aspergillus-dominated enterotypes decreased, and the proportion of Candida-dominated enterotypes remained relatively stable. The within-sample α diversity (phylogenetic diversity and richness) significantly decreased from T1 to T3, while the Shannon Index showed little change. Moreover, the alterations in the within-sample α diversity during pregnancy were very similar between women who gave birth to healthy infants and those who had preterm, low birthweight or macrosomia infants. Although dietary factors contributed to the gut mycobiome compositional variation across participants, there is no significant associations between changes in the consumption of eight main food groups from T1 to T3 and decreased richness
Dynamics of the individual gut fungal genus during pregnancy in the longitudinal subcohort
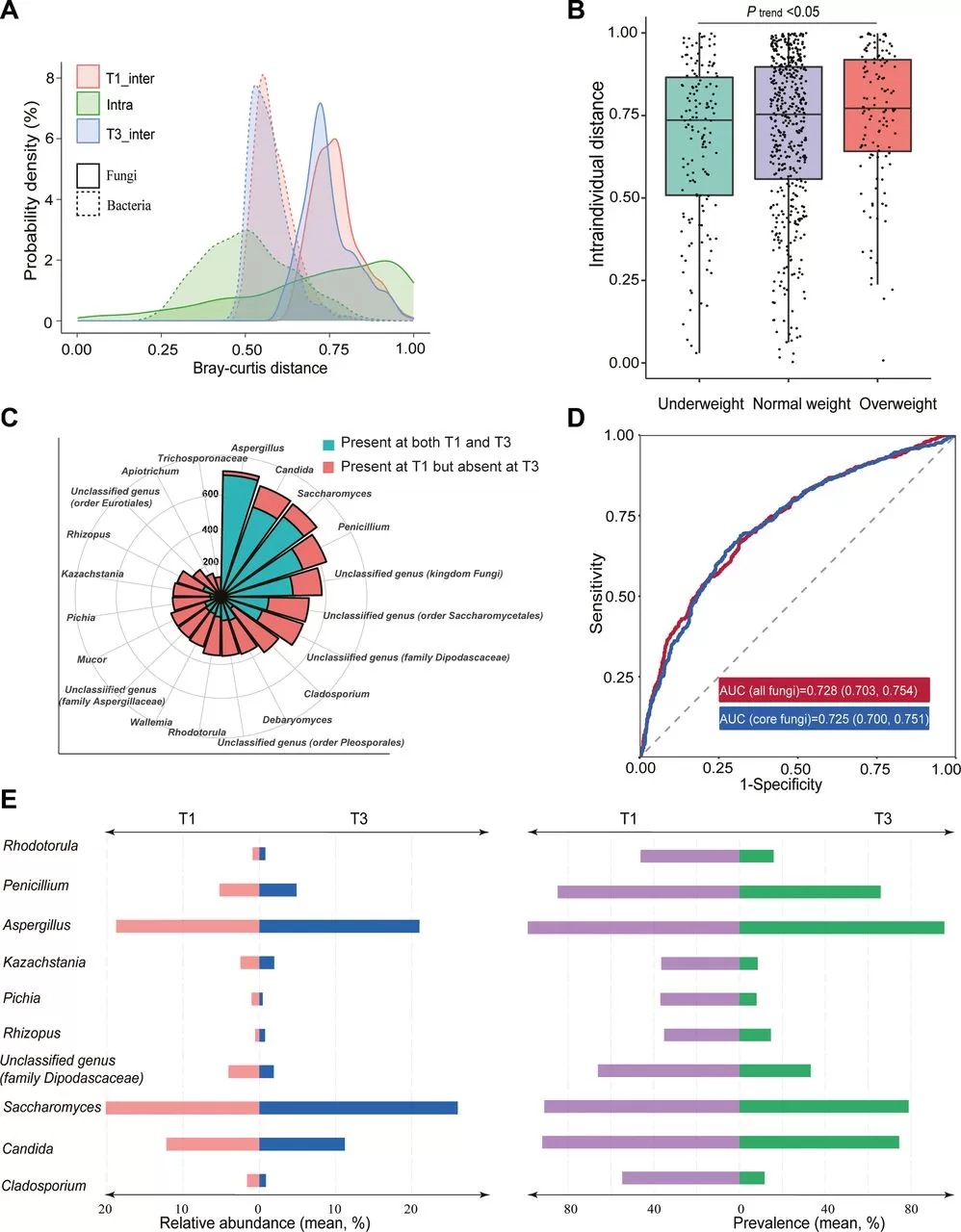
The extent of intraindividual shift in the composition was further profiled in the context of interindividual variation. The results showed there was a wide range of intraindividual Bray-Curtis distance from T1 to T2 or T1 to T3, most of which were even larger than the mean interindividual distance at a single time point. In contrast, the gut bacterial composition showed much less variation during pregnancy. Participants who experienced shifts in fungal enterotypes displayed significantly greater changes in gut mycobiome composition. Additionally, women who were overweight or obese before pregnancy experienced more significant gut fungal variations from T1 to T3 compared to those who were underweight before pregnancy.
The study found significant changes in the composition of gut fungal genera among 750 pregnant women during pregnancy. Among the 410 fungal genera identified in the T1 samples, the average loss rate of 390 uncommon genera was as high as 97.6%, while the average loss rate of the 20 core genera was 55.7%, with Aspergillus, Candida, and Saccharomyces being the most stable. In the late pregnancy compared to early pregnancy, four core fungal genera (Aspergillus, Cladosporium, Penicillium, and Candida) significantly decreased. Machine learning algorithms could effectively distinguish between T1 and T3 samples, with the core genera playing a major role in the discrimination. The characteristics of gut fungal composition in T2 and T3 samples were very similar, indicating the stability of gut fungal composition in late pregnancy.
Key microbial functional pathways and host serum metabolites correlated with gut mycobiome
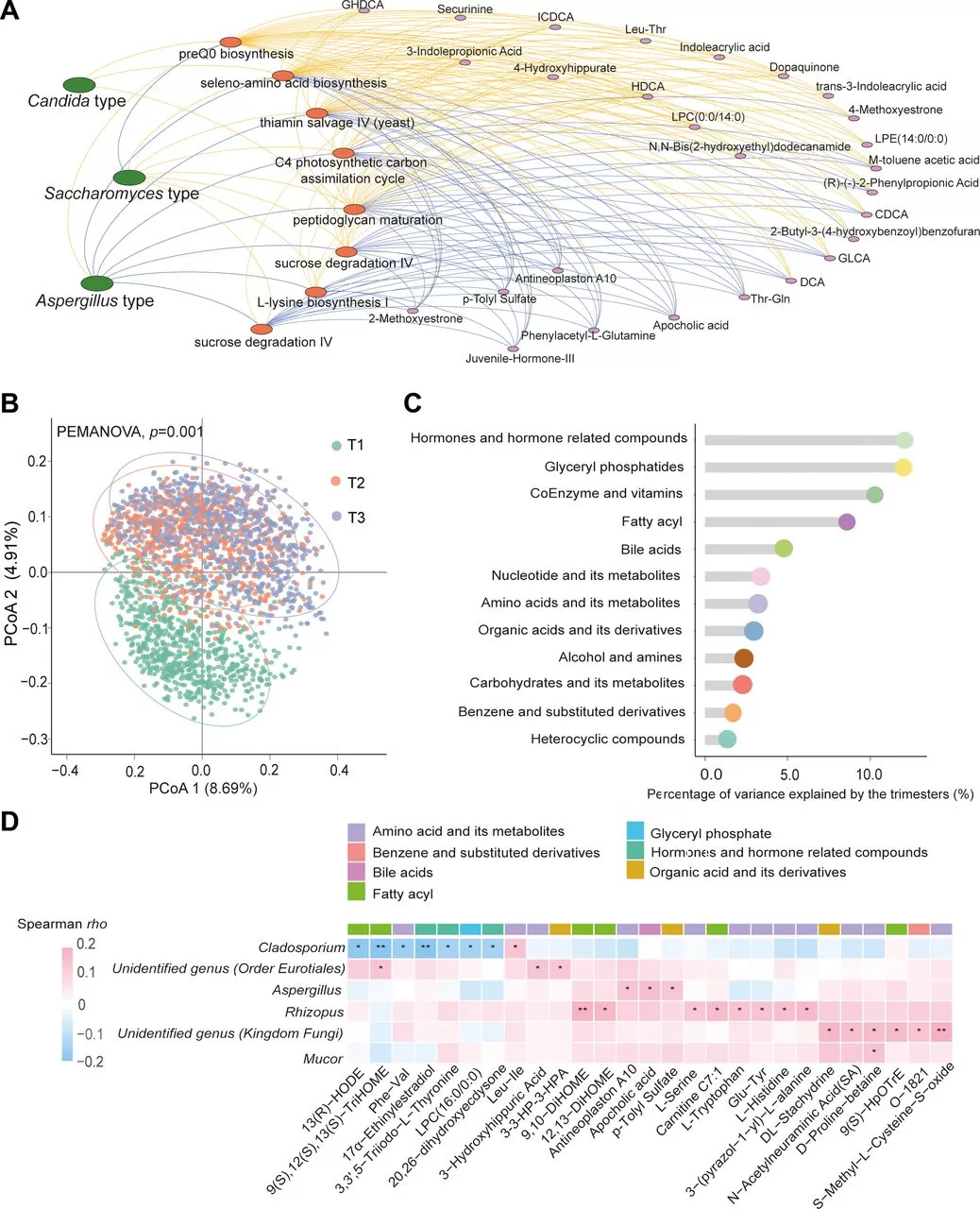
Based on metagenomic sequencing data, the study found that different types of gut fungi (such as Saccharomyces, Aspergillus, Candida) significantly influence the distribution of gut bacterial functional pathways and are associated with various serum metabolites. Specifically, the study identified eight functional pathways with relative abundance differences among different fungal types and found 95 significant pathways associated with 27 serum metabolites, most of which were bile acids, amino acid metabolites, and organic acid derivatives.
In the established subcohort, researchers analyzed metabolic changes by repeatedly measuring serum metabolites and explored their co-variation with gut fungi. The study quantified 794 metabolites, covering categories such as amino acids, lipids, nucleotides, and carbohydrates. Serum metabolome significantly changed across trimesters, especially from T1 to T2. The proportion of variance explained varied from 1.17% to 11.89%, with the largest variance observed in hormone-related metabolites. Correlation analysis revealed 30 co-variation relationships involving six fungal genera and 27 metabolites. Among them, four fungal genera were annotated at the genus level: Candida, Aspergillus, Rhizopus, and Mucor. Candida showed a negative correlation with seven amino acids or their metabolites, while Aspergillus, Rhizopus, and Mucor were positively correlated with different numbers of metabolites, with Rhizopus primarily associated with acylcarnitines.
Associations of gut mycobiome with pregnancy outcomes
The study found significant associations between core fungal genera and pregnancy complications during early pregnancy. Mucor was positively correlated with gestational diabetes mellitus (GDM), while Wallemia was positively correlated with pregnancy-induced hypertension (PIH). GDM increases the risk of macrosomia and preterm birth, while PIH increases the risk of low birth weight and preterm birth. The relative abundance of Mucor was independently associated with macrosomia risk and unrelated to GDM, indicating that they affect macrosomia risk through different pathways. Although overall changes in the gut mycobiome were not significantly associated with adverse birth outcomes, the trajectories of individual fungi (such as Mucor) were significantly associated with preterm birth.
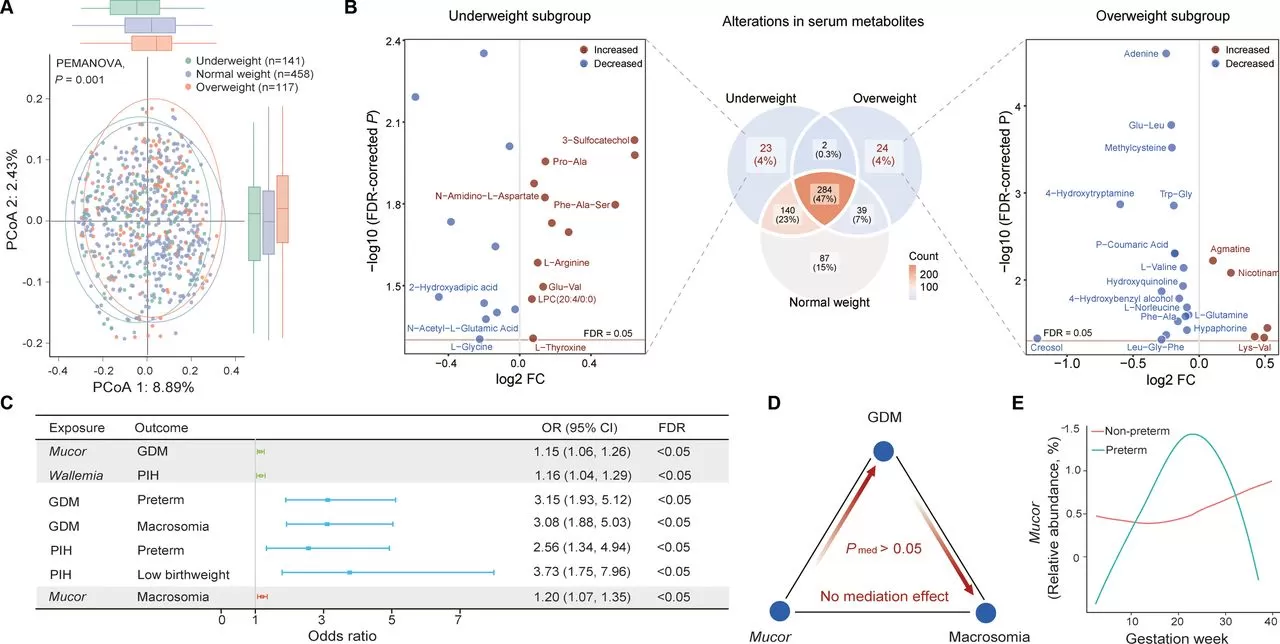
In summary, the study provides evidence for the dynamic nature of gut fungi in comparison to gut bacteria during pregnancy, revealing that prepregnancy overweight status may be a key determinant for this alteration. The study presents a landscape of the networks among the gut mycobiome, biological functionality, serum metabolites and pregnancy health, pinpointing the link between fungal genus Mucor and adverse pregnancy outcomes. This study also provides a reference database and resource for future investigation on the functional role of gut mycobiome in healthy pregnancy.
Tailored Metabolomics Solutions: Your Trusted Partner-MetwareBio
MetwareBio offered the widely-targeted metabolomics detection service for this research. For researchers embarking on projects akin to the study described, MetwareBio stands as a trusted ally in metabolomics detection. With a dedicated commitment to data quality and a nuanced understanding of the unique nature of each project, Metware offers tailored metabolomics services to suit diverse needs. Whether it's small-scale endeavors or large population studies, our workflows are adept at accommodating varying sample sizes and project scopes. Our extensive experience, reflected in over 4000 completed projects, underscores our proficiency in delivering reliable results. At MetwareBio, we prioritize collaboration, guiding researchers from sample extraction to data analysis to ensure their research goals are met with precision and efficiency. Please don't hesitate to reach out if you have any requirements or inquiries!
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


