Transcriptomics + Metabolomics in Color Research
Horticultural plants, encompassing fruits, vegetables, and flowers, exhibit a diverse range of color and quality traits, making these traits and their biochemical foundation a prominent research focus. The investigation of plant color delves into the mechanisms responsible for the variation in coloration observed in plant fruits, leaves, and flowers, attributed to differences in pigment accumulation. Plant colors can be categorized into four principal groups, based on the chemical structures of their metabolites: chlorophylls, carotenoids, flavonoids, and betalains.
Chlorophylls: These are lipophilic pigments primarily localized within plastids in plant cells and encompass two main types: chlorophyll-a and chlorophyll-b.
Carotenoids: Lipophilic pigments, primarily residing within plastids in plant cells, comprise well-known subtypes like orange carotenes, yellow xanthophylls, and red lycopene.
Flavonoids: Flavonoid pigments encompass a range of subcategories, including anthocyanins, flavone and chalcones. Anthocyanins, among these, exhibit the broadest distribution, with over 550 distinct varieties identified. They are primarily located within vacuoles and their color expression is closely linked to pH, appearing red under acidic conditions and blue under alkaline conditions.
Betalains: These pigments, characterized by hues of red or yellow, are principally found in the flowers, roots, stems, and leaves of plants. They are classified into two primary groups: red-purple betacyanins and yellow-orange betaxanthins. Notably, betalains are predominantly observed in plants of the Caryophyllales order.
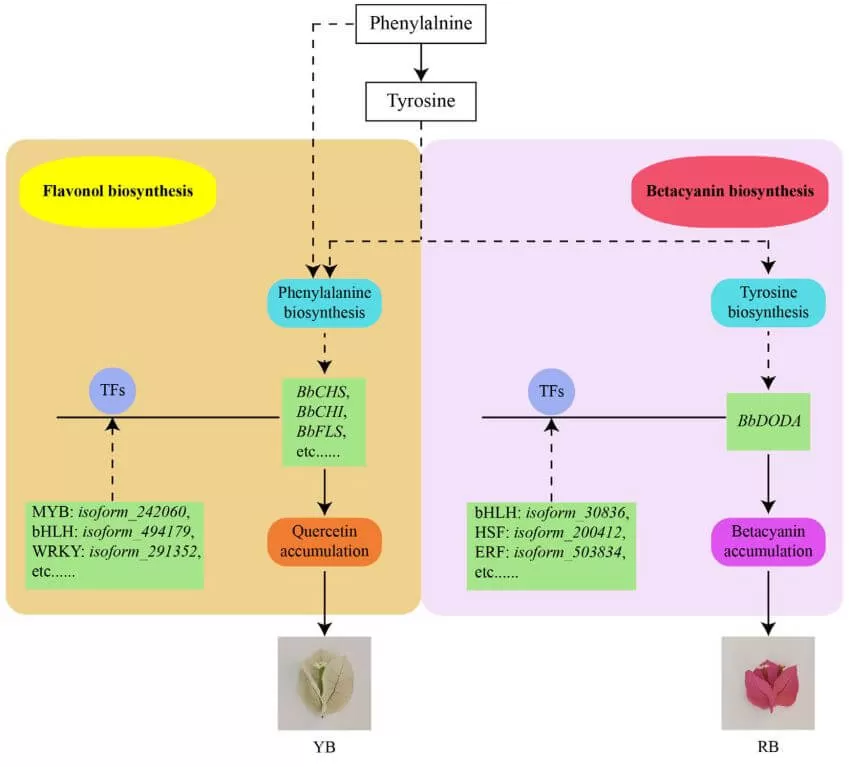 Bougainvillea is a typical tropical flower of great ornamental value due to its colorful bracts. A recent paper 'Integrated metabolome, full-length sequencing, and transcriptome analyses unveil the molecular mechanisms of color formation of the canary yellow and red bracts of Bougainvillea 3 buttiana 'Chitra'' which published in Plant Journal has revealed the molecular mechanism behind color formation in bougainvillea. This research conducted metabolome analysis, transcriptome analysis, and multi-flux full-length sequencing in two color bracts of Bougainvillea X buttiana 'Chitra' to investigate the significantly differential metabolites (SDMs) and differentially expressed genes (DEGs). Overall, 261 SDMs, including 62 flavonoids and 26 alkaloids, were detected, and flavonols and betalains were significantly differentially accumulated among the two bracts. RNA-seq findings revealed that 3610 DEGs were identified between two bracts. Co-expression analysis demonstrated that the DEGs and SDMs involved in flavonol metabolism (such as CHS, CHI, F3H, FLS, CYP75B1, kaempferol, and quercetin) and betacyanin metabolism (DODA, betanidin, and betacyanins) were the main contributors for the canary yellow and red bract formation, respectively. Moreover, several putative transcription factors (TFs) might interact with the promoters of the genes mentioned above. The expression profiles of the putative TFs displayed that they may positively and negatively regulate the structural genes’ expression profiles. The data revealed a potential regulatory network between important genes, putative TFs, and metabolites in the flavonol and betacyanin biosynthesis of Bougainvillea X buttiana ‘Chitra’ bracts. These findings will serve as a rich genetic resource for future studies that could create new color bracts.
Bougainvillea is a typical tropical flower of great ornamental value due to its colorful bracts. A recent paper 'Integrated metabolome, full-length sequencing, and transcriptome analyses unveil the molecular mechanisms of color formation of the canary yellow and red bracts of Bougainvillea 3 buttiana 'Chitra'' which published in Plant Journal has revealed the molecular mechanism behind color formation in bougainvillea. This research conducted metabolome analysis, transcriptome analysis, and multi-flux full-length sequencing in two color bracts of Bougainvillea X buttiana 'Chitra' to investigate the significantly differential metabolites (SDMs) and differentially expressed genes (DEGs). Overall, 261 SDMs, including 62 flavonoids and 26 alkaloids, were detected, and flavonols and betalains were significantly differentially accumulated among the two bracts. RNA-seq findings revealed that 3610 DEGs were identified between two bracts. Co-expression analysis demonstrated that the DEGs and SDMs involved in flavonol metabolism (such as CHS, CHI, F3H, FLS, CYP75B1, kaempferol, and quercetin) and betacyanin metabolism (DODA, betanidin, and betacyanins) were the main contributors for the canary yellow and red bract formation, respectively. Moreover, several putative transcription factors (TFs) might interact with the promoters of the genes mentioned above. The expression profiles of the putative TFs displayed that they may positively and negatively regulate the structural genes’ expression profiles. The data revealed a potential regulatory network between important genes, putative TFs, and metabolites in the flavonol and betacyanin biosynthesis of Bougainvillea X buttiana ‘Chitra’ bracts. These findings will serve as a rich genetic resource for future studies that could create new color bracts.
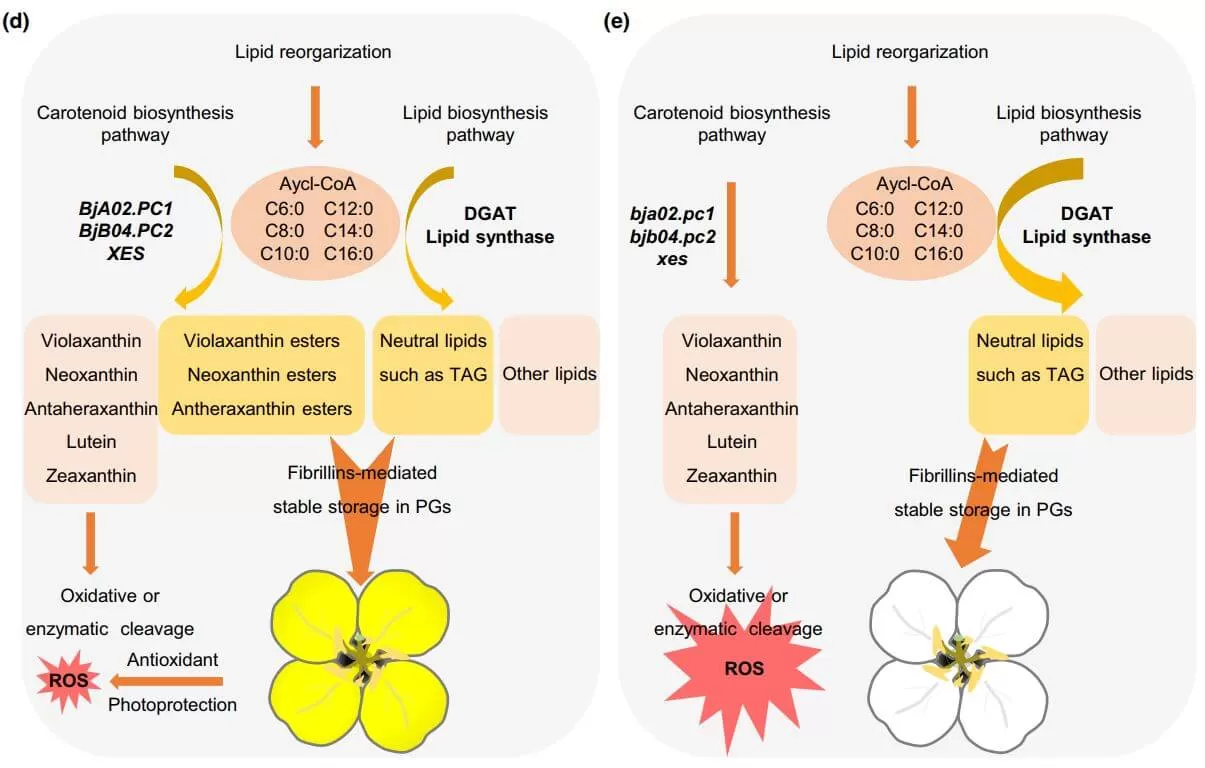 Another recent paper 'Xanthophyll esterases in association with fibrillins control the stable storage of carotenoids in yellow flowers of rapeseed (Brassica juncea)' which published in New Phytologist has identified a previously unknown carotenoid storage pathway, while offering unique opportunities for improving the stability, deposition, and bioavailability of carotenoids. The author identified two homologous genes, BjA02. PC1 and BjB04. PC2, belonging to the esterase/lipase/thioesterase (ELT) family of acyltransferases. They showed BjPCs in association with fibrillin gene BjFBN1b control the stable storage of carotenoids in yellow flowers of Brassica juncea. Through genetic, high-resolution mass spectrometry and transmission electron microscopy analyses, the study demonstrated that both BjA02. PC1 and BjB04. PC2 can promote the accumulation of esterified xanthophylls, facilitating the formation of carotenoid-enriched plastoglobules (PGs) and ultimately producing yellow pigments in flowers. The elimination of BjPCs led to the redirection of metabolic flux from xanthophyll ester biosynthesis to lipid biosynthesis, resulting in white flowers for B. juncea. Moreover, the author genetically verified the function of two fibrillin genes, BjA01. FBN1b and BjB05. FBN1b, in mediating PG formation and demonstrated xanthophyll esters must be deposited in PGs for stable storage. The authors propose a model of how BjPCs and BjFBN1b regulate the stable storage of carotenoids, resulting in the yellow coloration of flowers.
Another recent paper 'Xanthophyll esterases in association with fibrillins control the stable storage of carotenoids in yellow flowers of rapeseed (Brassica juncea)' which published in New Phytologist has identified a previously unknown carotenoid storage pathway, while offering unique opportunities for improving the stability, deposition, and bioavailability of carotenoids. The author identified two homologous genes, BjA02. PC1 and BjB04. PC2, belonging to the esterase/lipase/thioesterase (ELT) family of acyltransferases. They showed BjPCs in association with fibrillin gene BjFBN1b control the stable storage of carotenoids in yellow flowers of Brassica juncea. Through genetic, high-resolution mass spectrometry and transmission electron microscopy analyses, the study demonstrated that both BjA02. PC1 and BjB04. PC2 can promote the accumulation of esterified xanthophylls, facilitating the formation of carotenoid-enriched plastoglobules (PGs) and ultimately producing yellow pigments in flowers. The elimination of BjPCs led to the redirection of metabolic flux from xanthophyll ester biosynthesis to lipid biosynthesis, resulting in white flowers for B. juncea. Moreover, the author genetically verified the function of two fibrillin genes, BjA01. FBN1b and BjB05. FBN1b, in mediating PG formation and demonstrated xanthophyll esters must be deposited in PGs for stable storage. The authors propose a model of how BjPCs and BjFBN1b regulate the stable storage of carotenoids, resulting in the yellow coloration of flowers.
If you work with plant colors and are interested in conducting metabolomics or multi-omics, we offer the following services:
- Widely-Targeted Metabolomics for Plants ( >30,000 plant metabolites )
- Flavonoids Metabolomics (>3,700 flavonoids)
- Targeted Anthocyanin Assay
- Targeted Carotenoid Assay
Explore more in our related blog posts:
- Metabolomics of Plant Pigmentation: Exploring the Rich Tapestry of Natural Colors
- Plant Color Metabolomics (1/2)
- Plant Color Metabolomics (2/2)
Feel free to reach out if you have any questions or if you'd like further information.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


