ATP vs ADP: Structure, Energy, and What the Ratio Really Tells You
Adenosine triphosphate (ATP) and adenosine diphosphate (ADP) are central to how cells store and release energy. Understanding their differences—and how the ATP/ADP ratio reflects cellular energy status—is key to modern biology and disease research. In this article, we explore what sets ATP and ADP apart, how they cycle within the cell, and why their balance matters in health, stress, and disease. We’ll also touch on how scientists measure these metabolites and what their levels reveal about cellular function.
Understanding ATP and ADP
What is ATP?
ATP, or adenosine triphosphate, is a nucleotide made of three main parts: an adenine base, a ribose sugar, and three phosphate groups linked in a chain. The bonds between the phosphate groups, especially the last two known as phosphoanhydride bonds, store substantial amounts of chemical energy. When cells need to perform work—such as contracting a muscle, transporting ions, or synthesizing macromolecules—ATP is hydrolyzed into ADP (adenosine diphosphate) and an inorganic phosphate (Pi), releasing usable energy. This is why ATP is often called the "energy currency" of the cell. Read more on Adenosine Triphosphate (ATP): The Key to Cellular Energy Metabolism.
What is ADP?
ADP, or adenosine diphosphate, has a similar structure to ATP but with only two phosphate groups. It is the product of ATP hydrolysis, and while it carries less energy, it plays a critical role in the cellular energy cycle. ADP is continuously phosphorylated back into ATP through metabolic pathways such as oxidative phosphorylation and substrate-level phosphorylation. This regeneration allows cells to maintain a high ATP/ADP ratio and sustain energy-dependent processes.
ATP vs ADP — Key Differences at a Glance
ATP and ADP differ structurally and functionally in ways that are central to how cells manage energy. The table below highlights their core differences in phosphate number, energy content, roles, and how they interconvert.
Table 1: Comparison of ATP and ADP.
|
Feature |
ATP (Adenosine Triphosphate) |
ADP (Adenosine Diphosphate) |
|
Phosphate Groups |
Three |
Two |
|
Structure |
Adenine + ribose + 3 phosphates (C₁₀H₁₆N₅O₁₃P₃) |
Adenine + ribose + 2 phosphates (C₁₀H₁₅N₅O₁₀P₂) |
|
Molecular Weight |
~507.18 g/mol |
~427.20 g/mol |
|
Energy Content |
High energy — releases ~30.5 kJ/mol when hydrolyzed |
Lower energy — contains one high-energy bond |
|
Role in Cell |
Main energy source for cellular processes |
Intermediate — recycled back into ATP |
|
Formation |
Phosphorylation of ADP (oxidative/substrate-level) |
Hydrolysis of ATP; also from AMP via kinase |
|
Interconversion |
ATP → ADP + Pi + Energy |
ADP → ATP (with energy input) |
Understanding these differences is key to interpreting the ATP/ADP ratio in cellular metabolism, disease research, and energy profiling.
ATP/ADP Ratio and Adenylate Energy Charge (AEC)
Cells constantly convert ATP to ADP as they use energy, then regenerate ATP to restore balance. The ATP/ADP ratio is a key indicator of cellular energy availability—higher ratios mean more power to fuel biological processes. In healthy cells, this ratio often exceeds 10:1, keeping ATP hydrolysis highly exergonic and efficient.
To capture a broader view of energy status, scientists use the adenylate energy charge (AEC), which reflects the relative levels of ATP, ADP, and AMP:
AEC = ( [ATP] + 0.5 × [ADP] ) / ( [ATP] + [ADP] + [AMP] )
AEC ranges from 0 (all AMP) to 1 (all ATP), and most viable cells maintain a value between 0.7 and 0.95. AEC below 0.5 signals serious energy stress, potentially leading to metabolic failure or cell death.
Table 2. Typical AEC Values and Cellular Status
|
AEC Range |
Cellular State |
|
0.90 – 1.00 |
Optimal energy charge |
|
0.70 – 0.89 |
Mild energy stress |
|
0.50 – 0.69 |
Compromised metabolism |
|
Below 0.50 |
Critical energy depletion |
Cells respond to low AEC by activating AMP-activated protein kinase (AMPK), which conserves energy and stimulates ATP production. Monitoring ATP/ADP ratio and AEC offers valuable insight into metabolic health across biology, disease, and stress conditions.
How ATP and ADP Cycle Powers Cellular Energy
The ATP–ADP cycle allows cells to store and release energy efficiently. ATP is hydrolyzed to ADP when energy is needed, and ADP is continuously regenerated back to ATP. This reversible process powers nearly every biological activity, from movement to metabolism.
ATP Hydrolysis: Energy Release for Cellular Work
ATP hydrolysis releases ~30.5 kJ/mol of energy, fueling essential processes like:
• Muscle contraction (myosin motors)
• Ion transport (e.g. Na⁺/K⁺-ATPase)
• Biosynthesis of proteins, DNA, lipids
• Signal transduction via phosphorylation
Note: −30.5 kJ/mol refers to standard biochemical conditions (ΔG°′); actual values vary with Mg²⁺, pH, and ionic strength.
The reaction:
ATP + H₂O → ADP + Pi + Energy (ΔG°′ ≈ −30.5 kJ/mol)
This exergonic reaction provides immediate, usable energy. Cells maintain a high ATP/ADP ratio to ensure the energy release remains efficient.
ADP to ATP Conversion: Oxidative Phosphorylation vs. Substrate-Level Phosphorylation
Recharging ADP to ATP requires an input of energy to add a phosphate back onto ADP. Cells have two primary ways to do this:
|
Pathway |
Oxidative Phosphorylation |
Substrate-Level Phosphorylation |
|
Location |
Mitochondria |
Cytosol / Mitochondrial matrix |
|
Oxygen Requirement |
Yes |
No |
|
ATP Yield (per glucose) |
High (~30–32 ATP) |
Low (2–4 ATP) |
|
Key Enzyme |
ATP Synthase |
Pyruvate kinase, succinyl-CoA synthetase |
|
Speed / Context |
Efficient but slower |
Rapid response, low O₂ conditions |
These mechanisms work together. For example, muscle cells rely on glycolysis (substrate-level) for quick ATP, while OXPHOS catches up during sustained activity.
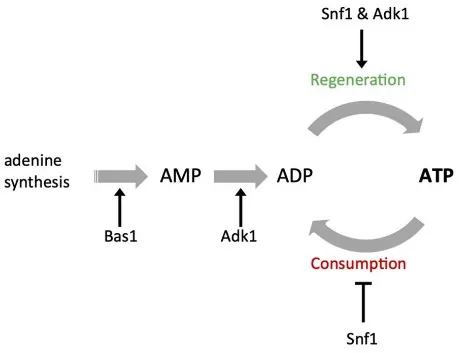
Models for ATP homeostasis and its role in proteostasis
Source: Takaine M, Imamura H, Yoshida S. “High and stable ATP levels prevent aberrant intracellular protein aggregation in yeast,” eLife (2022) 11:e67659. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Where ATP → ADP Powers Biology
ATP hydrolysis (ATP → ADP + Pi) drives essential cellular work. In muscle contraction, myosin uses ATP for the power stroke and detachment. Ion transport relies on ATP (e.g., Na⁺/K⁺-ATPase, Ca²⁺-ATPases) to maintain gradients for excitability. Biosynthesis consumes ATP equivalents to charge tRNA and fuel macromolecule assembly. Signal transduction uses ATP as a phosphate donor for protein phosphorylation and as the precursor of cAMP. These processes demand a high ATP/ADP ratio; when ATP falls and ADP rises, performance degrades and AMPK conserves energy. In short, ATP vs ADP determines whether cells can sustain contraction, transport, synthesis, and signaling in real time.
ATP/ADP Ratio Matters in Human Disease: Disturbances in ATP supply quickly appear as a falling ATP/ADP ratio and lower AEC. Tracking this ratio quantifies bioenergetic stress and helps judge whether interventions truly restore ATP generation.
Cancer Metabolism and the ATP/ADP Ratio
Tumors reprogram bioenergetics to sustain growth. Glycolysis-dominant cancers often tolerate a lower cytosolic ATP/ADP ratio, relieving feedback inhibition and driving high glycolytic flux; oxidative-competent clones maintain high ATP output from both glycolysis and OXPHOS to fuel invasion and therapy resistance. In practice, quantifying ATP, ADP, and AMP alongside central-carbon metabolites reveals whether glycolysis, TCA, or the electron transport chain constrains energy supply. Recent high-impact studies show that inhibiting ATP synthase or collapsing the proton motive force selectively impairs aggressive phenotypes while sparing less bioenergetic subpopulations—highlighting the ratio as both a readout and potential vulnerability. For discovery and patient-derived models, pairing ATP/ADP and AEC with isotope-tracing offers the clearest picture of how cancers finance proliferation and how to sensitize them to standard therapy.
Ischemia–Reperfusion Injury and the ATP/ADP Ratio (Heart/Brain)
When oxygen delivery drops, oxidative phosphorylation stalls; ATP falls and ADP rises, collapsing the ATP/ADP ratio and AEC. Ion pumps fail, Ca²⁺ overload ensues, and cells enter energy crisis. Reperfusion restores oxygen but often adds ROS bursts and transient mitochondrial dysfunction, delaying ATP recovery. Contemporary translational work maps ATP/ADP to distinguish necrotic core (profound charge loss) from salvageable penumbra (partially preserved charge). Interventions that stabilize mitochondria—temperature control, complex I modulation, adenylate kinase support, or optimized substrate delivery—tend to preserve or accelerate ratio recovery. In preclinical heart models, protecting adenine nucleotide pools limits adenosine washout and improves contractile recovery; in stroke, faster normalization of ATP/ADP in penumbra predicts better outcomes. Thus, the ratio functions as a pragmatic biomarker for injury severity and treatment efficacy.
Sepsis, Critical Illness, and the ATP/ADP Ratio
Sepsis couples inflammatory signaling with mitochondrial dysfunction and microcirculatory heterogeneity. Even with adequate oxygen, tissues can show low ATP/ADP ratios—a signature of impaired oxidative phosphorylation and elevated energy demand. High-level clinical studies report lower muscle ATP and energy charge in non-survivors, aligning bioenergetic failure with organ dysfunction. Mechanistically, inflammatory mediators inhibit respiratory complexes, increase proton leak, and divert substrates, depressing ADP→ATP conversion efficiency. Emerging “metabolic resuscitation” strategies—redox support, mitochondrial coupling enhancers, tailored nutrition—now use restoration of the ATP/ADP ratio in immune cells or muscle as functional endpoints. A transient hypometabolic adaptation may be protective; the risk is a persistent inability to re-establish a high ratio during recovery.
Neurodegeneration and the ATP/ADP Ratio
Neurons operate near their energetic ceiling. Chronic oxidative stress, defective mitophagy, or respiratory-chain damage reduces ADP→ATP conversion, depressing the ATP/ADP ratio and AEC. Reviews and recent studies document oxidative modifications of ATP synthase and other OXPHOS components in Alzheimer’s and Parkinson’s disease, with early shifts in neuronal ADP/ATP preceding overt degeneration. Functionally, a lower ratio weakens ion-pump reserve, impairs vesicle cycling, and limits plasticity; activity bursts further deplete ATP, triggering AMPK and energy-conserving programs at the expense of function. Therapeutic work therefore aims to stabilize the ratio by enhancing mitochondrial biogenesis and coupling, buffering redox stress, or providing alternative fuels (e.g., ketones). In patient-derived neurons and in vivo, combining ATP/ADP quantitation with electrophysiology links metabolic reserve to circuit performance.
Measuring ATP and ADP
Accurate quantification of ATP and ADP underpins bioenergetics research. The ATP/ADP ratio is routinely assessed with three practical approaches; choice depends on throughput, matrix, and whether absolute or real-time data are required.
Bioluminescent (luciferase) assays are the fastest route for ATP and ATP/ADP ratio screening. Kits read ATP directly, then enzymatically convert ADP to ATP for a second read; the difference yields ADP. They’re ultra-sensitive and plate-friendly, ideal for viability screens and rapid kinetic checks. Careful quenching is essential to prevent post-lysis turnover. (Minimal note: Use rapid cold/acidic extraction or immediate enzyme quench to limit artifactual interconversion.)
HPLC or LC–MS/MS provides absolute quantitation of ATP, ADP, and AMP—often alongside central-carbon metabolites—enabling AEC (adenylate energy charge) calculation. With proper rapid extraction, this is the gold standard for tissues, primary cells, and flux studies (including isotope tracing). It distinguishes closely related nucleotides and scales to cohort studies.
Genetically encoded sensors / ^31P-NMR support real-time or non-invasive readouts. Fluorescent ATP:ADP sensors (e.g., Perceval variants) map subcellular dynamics in living cells, while ^31P-NMR monitors high-energy phosphates in intact tissues. These are powerful for mechanism, less suited to routine quantitation.
Quick comparison:
|
Method |
Measures |
Strengths |
Considerations |
|
Bioluminescence (ATP/ADP kits) |
ATP, ADP (ratio) |
Fast, high-throughput, sensitive |
Requires strict quenching/controls |
|
HPLC / LC–MS/MS |
ATP, ADP, AMP (absolute) |
Specific, multiplexed, AEC-ready |
More setup; rapid extraction needed |
|
Genetically encoded sensors |
ATP:ADP dynamics |
Live-cell, spatial/temporal data |
Calibration; not absolute amounts |
|
^31P-NMR |
High-energy phosphates |
Non-invasive tissue readouts |
Specialized hardware, lower S/N |
For discovery and validation, a common workflow pairs rapid ATP/ADP screening by luminescence with confirmatory LC–MS/MS for absolute values and AEC.
FAQ
Q: What’s the difference between ATP and ADP?
A: ATP (triphosphate) carries more usable energy than ADP (diphosphate). Cells hydrolyze ATP → ADP + Pi to power work, then recycle ADP back to ATP.
Q: Why does the ATP/ADP ratio matter?
A: It’s a live readout of cellular energy; a low ratio signals stress and triggers AMPK to conserve energy and restore ATP.
Q: What is adenylate energy charge (AEC)?
A: AEC = ( [ATP] + 0.5 × [ADP] ) / ( [ATP] + [ADP] + [AMP] ). Healthy cells ~0.70–0.95; <0.50 indicates critical depletion.
Q: How is ADP converted back to ATP?
A: Mainly by oxidative phosphorylation (ATP synthase) and substrate-level phosphorylation (e.g., glycolysis).
Q: How do you measure ATP/ADP?
A: Fast screens use luciferase ATP/ADP kits; LC–MS/MS gives absolute ATP, ADP, AMP for AEC; sensors or ^31P-NMR enable real-time/non-invasive reads.
Conclusion
ATP vs ADP explains how cells store and spend energy, while the ATP/ADP ratio and AEC quantify that balance. Tracking these metrics clarifies physiology, stress responses, and disease mechanisms—from ischemia to cancer and neurodegeneration.
Looking to quantify ATP, ADP (±AMP) and related pathways? Our LC–MS/MS metabolomics services provide robust, research-grade readouts to support your study design and discovery.
Reference
- Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Biochemistry. 1968;7(11):4030-4034. doi:10.1021/bi00850a033.
- Maldonado EN, Lemasters JJ. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 2014;19(Pt A):78-84. doi:10.1016/j.mito.2014.09.002.
- Fiorillo M, Sotgia F, Lisanti MP. High ATP production fuels cancer drug resistance and metastasis: Implications for mitochondrial ATP depletion therapy. Front Oncol. 2021;11:740720. doi:10.3389/fonc.2021.740720.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121-1135. doi:10.1056/NEJMra071667.
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229-317. doi:10.1016/B978-0-12-394309-5.00006-7.
- Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219-223. doi:10.1016/S0140-6736(02)09452-6.
- Wasyluk W, Zwolak A. Metabolic alterations in sepsis. J Clin Med. 2021;10(11):2412. doi:10.3390/jcm10112412.
- Ebanks B, Chakrabarti L. Mitochondrial ATP synthase is a target of oxidative stress in neurodegenerative diseases. Front Mol Biosci. 2022;9:854321. doi:10.3389/fmolb.2022.854321.
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787-795. doi:10.1038/nature05292.
- Dunn J, Grider MH. Physiology, Adenosine Triphosphate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535365/
- Junge W, Nelson N. ATP synthase. Annu Rev Biochem. 2015;84:631-657. doi:10.1146/annurev-biochem-060614-034124.
- Boyer PD. The ATP synthase—a splendid molecular machine. Annu Rev Biochem. 1997;66:717-749. doi:10.1146/annurev.biochem.66.1.717.
- Tantama M, Martínez-François JR, Mongeon R, Yellen G. Imaging energy status in live cells with a fluorescent biosensor of the ATP:ADP ratio. Nat Commun. 2013;4:2550. doi:10.1038/ncomms3550.
- Takaine M, Imamura H, Yoshida S. High and stable ATP levels prevent aberrant intracellular protein aggregation in yeast. eLife. 2022;11:e67659. doi:10.7554/eLife.67659.
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251-262. doi:10.1038/nrm3311.
- Nicholls DG, Ferguson SJ. Bioenergetics 4. Amsterdam: Academic Press; 2013. doi:10.1016/C2010-0-66146-3.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


