Isoprenoids Explained: Mevalonate (MVA) vs MEP Pathways, CoQ10 and Dolichol in Health and Disease
Isoprenoids—also known as terpenoids—are the most structurally diverse family of natural compounds, all biosynthesized from the universal five-carbon precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). These C₅ units are produced via two distinct pathways: the mevalonate (MVA) pathway, predominant in animals and fungi, and the methylerythritol phosphate (MEP) pathway, used by bacteria and plastids in plants. Together, these pathways underpin a vast metabolic landscape, giving rise to coenzyme Q (ubiquinone) for mitochondrial respiration, dolichol phosphate for protein glycosylation, and prenylated proteins essential in signal transduction. In this article, we dissect the logic of isoprenoid biosynthesis, compare the MVA vs. MEP routes, and explore the biological and clinical relevance of their downstream products. Through recent findings, analytical approaches, and disease insights, we outline how this ancient chemical logic continues to shape modern biomedicine and biotechnology.
What Are Isoprenoids? Structure, Isoprene Units and Modular Assembly
Isoprenoids are defined by their construction from repeating five-carbon units known as isoprene (C₅H₈). This structural pattern, first articulated as the “isoprene rule” by chemists like Wallach and Ruzicka in the early 20th century, forms the basis for classifying natural compounds as terpenes or terpenoids. These units are enzymatically assembled via head-to-tail condensation of two activated precursors: isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Depending on the number of C₅ repeats, isoprenoids are categorized into classes such as monoterpenes (C₁₀), sesquiterpenes (C₁₅), diterpenes (C₂₀), and polyisoprenoids. Examples range from menthol and limonene to cholesterol, ubiquinone, and natural rubber. Despite their vast structural and functional diversity, all isoprenoids share this modular architecture. Historically isolated from plant resins and essential oils, isoprenoids are now recognized as ubiquitous in all domains of life, serving roles in metabolism, signaling, membrane dynamics, and more.
Table 1. Representative Isoprenoid Classes and Functions
|
Isoprenoid Class |
Carbon Backbone |
Examples |
Biological Function |
|
Monoterpenes |
C₁₀ |
Menthol, Limonene |
Volatile signaling; antimicrobial defense (plants) |
|
Sesquiterpenes |
C₁₅ |
Farnesol, Nerolidol |
Precursors for hormones, pheromones |
|
Diterpenes |
C₂₀ |
Phytol, Taxadiene |
Chlorophyll tail; hormone and drug precursors |
|
Triterpenes |
C₃₀ |
Squalene, Lanosterol |
Cholesterol biosynthesis; steroid precursors |
|
Tetraterpenes |
C₄₀ |
β-Carotene, Lutein |
Photosynthesis pigments; vitamin A source |
|
Polyisoprenoids |
>C₄₀ |
Dolichol, Natural rubber |
Protein glycosylation; structural materials |
|
Quinones |
Variable |
Coenzyme Q₁₀ (Ubiquinone), Phylloquinone |
Mitochondrial electron transport; vitamin K activity |
|
Prenylated Proteins |
C₁₅/C₂₀ tails |
Farnesylated Ras, Geranylgeranylated Rho |
Membrane anchoring; intracellular signal transduction |
Biosynthesis of Isoprenoids: Mevalonate (MVA) vs MEP Pathways
Compartmentation of Isoprenoid Biosynthesis
Isoprenoid precursors—isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP)—are synthesized via two evolutionarily distinct routes: the mevalonate (MVA) pathway and the methylerythritol phosphate (MEP) pathway. In mammals, fungi, and archaea, the cytosolic MVA pathway predominates. Plants, by contrast, operate both: the MVA pathway in the cytosol (for sterols, sesquiterpenes, etc.) and the MEP pathway in plastids (for carotenoids, gibberellins, etc.), enabling metabolic flexibility and differential regulation of carbon flux.
Key Enzymes and Branch Points
The MVA pathway begins with acetyl-CoA condensation catalyzed by ACAT and HMG-CoA synthase, culminating in HMG-CoA reductase (HMGCR)—a rate-limiting step targeted by statins. The pathway generates IPP, which is isomerized to DMAPP. In parallel, the MEP pathway initiates from pyruvate and glyceraldehyde-3-phosphate, catalyzed by DXS and DXR (IspC), followed by several intermediates to reach HMBPP, which is converted into IPP and DMAPP by IspH. Farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP) act as critical branch nodes toward ubiquinone, dolichol, and prenylated proteins.
Crosstalk and Pharmacological Targeting
In plants and some microbes, metabolic crosstalk enables IPP/DMAPP exchange between MVA and MEP compartments. This redundancy is adaptive but also therapeutically exploitable: the absence of the MEP pathway in humans renders its enzymes—especially DXR—attractive antimicrobial targets. Agents like fosmidomycin, a DXR inhibitor, have shown efficacy against Plasmodium falciparum and Gram-negative bacteria. Meanwhile, statin-mediated MVA inhibition may have off-target effects by limiting downstream CoQ and protein prenylation, impacting mitochondrial and signaling functions.
Metabolism & Downstream Branches of Isoprenoids
Coenzyme Q (Ubiquinone) Biosynthesis & Redox Readouts
Coenzyme Q (ubiquinone) is a key mitochondrial electron carrier built from a polyisoprenoid tail and a benzoquinone head. It shuttles electrons from complexes I/II to III and acts as a lipid-phase antioxidant. Synthesized via the mevalonate pathway, CoQ₁₀ requires the multi-enzyme COQ complex (COQ2–COQ9). Mutations in these genes cause primary CoQ deficiency, leading to neuromuscular and renal disorders.
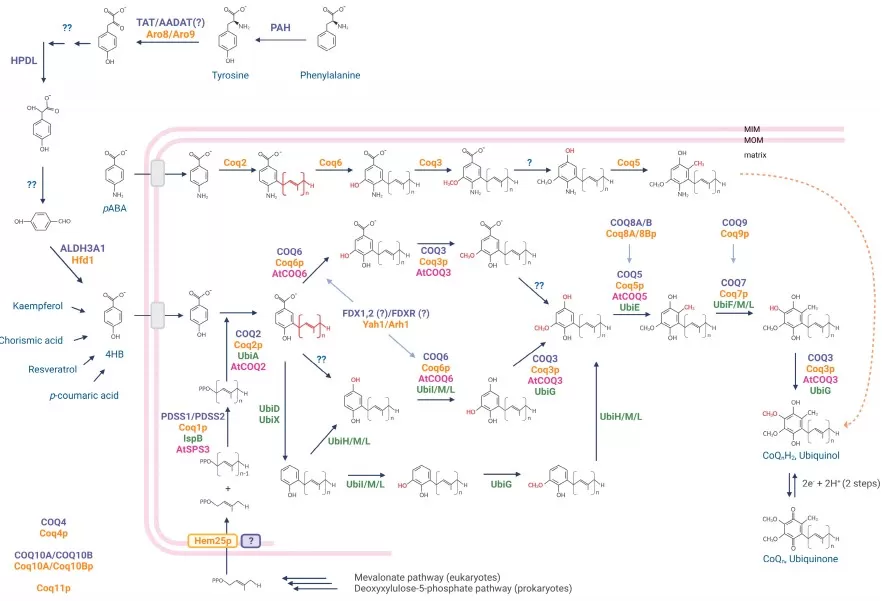
figure coq biosynthesis redox staiano 2023
Source: Staiano C, García-Corzo L, Mantle D, et al. “Biosynthesis, deficiency, and supplementation of coenzyme Q,” Antioxidants (2023) 12(7):1469. Figure 1. Licensed under CC BY 4.0. DOI: 10.3390/antiox12071469. No endorsement implied.
CoQ levels and redox state are measured using LC–MS/MS, distinguishing ubiquinone and ubiquinol forms. Internal standards (e.g., CoQ₉ or d₉-CoQ₁₀) ensure analytical accuracy. Monitoring CoQ is important in statin-treated patients or mitochondrial disease. Emerging links between CoQ, ferroptosis, and FSP1-mediated antioxidant defense make ubiquinone biosynthesis increasingly relevant in cancer metabolism and oxidative stress research.
Dolichol Phosphate & N-Glycosylation
Dolichol biosynthesis has been revised to a three-step route: DHRSX first oxidizes polyprenol → polyprenal, SRD5A3 reduces polyprenal → dolichal, and DHRSX then reduces dolichal → dolichol. DHRSX resides in the pseudoautosomal regions of X/Y; missense variants define DHRSX-CDG, explaining N-glycosylation defects and correcting long-standing assumptions that SRD5A3 alone converts polyprenol to dolichol. The model is supported by human genetics and CHO Lec5/Lec9 rescue data.
Pathogenic variants in DHRSX, DHDDS, or NUS1 cause congenital disorders of glycosylation (CDG) with multisystem phenotypes. In practice, dolichol and its precursors can be quantified by lipidomics LC–MS (and in some settings GC–MS for long-chain polyisoprenols), with careful handling to address hydrophobicity and redox artefacts. Functionally, insufficient dolichol phosphate supply acts as a metabolic bottleneck linking isoprenoid flux to N-glycosylation failure and severe developmental disease.
Protein Prenylation: Farnesylation and Geranylgeranylation
Protein prenylation attaches farnesyl (C₁₅) or geranylgeranyl (C₂₀) lipids to GTPases like Ras, Rho, and Rab, enabling membrane targeting and signal transduction. These groups are derived from FPP and GGPP, key outputs of the mevalonate pathway.
FTase and GGTase enzymes catalyze prenylation. Dysregulation contributes to cancer (Ras hyperactivation), progeria (farnesylated progerin), and autoinflammatory diseases like MKD via defective Rab prenylation and NLRP3 activation. Statins also impair prenylation indirectly.
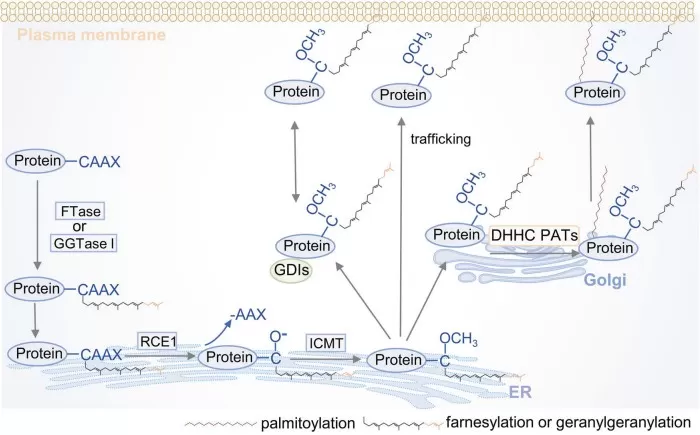
figures prenylation catalytic processyuan 2024
Source: Yuan Y, Li P, Li J, Zhao Q, Chang Y, He X. “Protein lipidation in health and disease,” Signal Transduction and Targeted Therapy (2024) 9:60. Figure 7. Licensed under CC BY 4.0. DOI: 10.1038/s41392-024-01759-7. No endorsement implied.
Prenylation assays combine LC–MS/MS with bioorthogonal probes or specific antibodies. With applications in oncology, inflammation, and microbial inhibition, protein prenylation remains a high-value pharmacological target.
Table 2: Farnesylation vs. Geranylgeranylation in Protein Prenylation
|
Feature |
Farnesylation |
Geranylgeranylation |
|
Isoprenoid donor |
FPP (C₁₅) |
GGPP (C₂₀) |
|
Catalyzing enzyme |
Farnesyltransferase (FTase) |
Geranylgeranyltransferase (GGTase I/II) |
|
Target proteins |
Ras, Lamin A |
Rho, Rab, Rac |
|
Functional effect |
Membrane localization |
Signal trafficking, vesicle fusion |
|
Therapeutic angle |
FTIs (e.g. lonafarnib) in cancer and progeria |
Statins, GGTIs in inflammation, oncology |
How to Measure Isoprenoids by Targeted Metabolomics and Lipidomics
Accurate measurement of isoprenoids underpins both basic biology and translational studies. Two complementary readouts are typically pursued: (i) absolute quantification by targeted LC–MS/MS and (ii) pathway dynamics by stable isotope tracing. These approaches cover hydrophobic neutral lipids (e.g., dolichol, ubiquinone/ubiquinol) and highly polar prenyl diphosphates (FPP/GGPP).
Targeted LC–MS/MS. Use matrix-matched calibration and stable isotope–labeled internal standards (e.g., d-labeled CoQ₁₀, ^13C-FPP/GGPP). For CoQ, monitor both ubiquinone and ubiquinol and report the redox ratio. To prevent artefactual oxidation of ubiquinol, (1) perform rapid, cold quenching at sampling, (2) spike a reduced, isotope-labeled internal standard at quench, (3) add antioxidants/metal chelators (e.g., BHT, EDTA), (4) use degassed solvents, amber glassware, minimal headspace, and (5) separate and analyze under conditions that preserve ubiquinol. For FPP/GGPP, employ ion-pair LC–MS/MS (e.g., tributylamine/TEA systems) or validated HILIC methods and negative-mode MRM transitions that target the pyrophosphate.
Stable isotope tracing. Substrate choice informs pathway partitioning: ^13C-mevalonate or ^13C-acetate/glucose preferentially probe the MVA route in the cytosol, whereas ^13C-glycerol/glucose (and pathway-specific precursors) inform MEP flux in plastids/bacteria. Acquire short time-courses, quantify labeled end-products (e.g., carotenoids, dolichols, CoQ) by LC–MS or GC–MS, and compute mass-isotopologue distributions (MID) with natural-abundance correction; report labeling into both pools and intermediates when feasible.
Pitfalls & QC. Hydrophobic analytes adsorb to plastics—prefer silanized/amber glass, add carrier, and validate recovery spikes. Control matrix effects (SPE cleanup, dilution), randomize batches with pooled QC every 10–12 injections, verify retention-time stability and ion-ratio criteria, and include extraction blanks. For long-chain polyisoprenoids, optimize extraction (e.g., propanol/IPA) and storage (cold, dark, low-O₂).
Together, targeted LC–MS/MS and stable isotope tracing yield reproducible concentration and flux readouts across CoQ redox, dolichol supply, and prenylation precursors (FPP/GGPP)—a practical toolkit for isoprenoid biosynthesis and disease-linked phenotyping.
Isoprenoid Pathways in Human Health and Disease
Isoprenoid metabolism underpins core processes like respiration, protein modification, and glycoprotein assembly. Consequently, genetic or pharmacological disruption of isoprenoid biosynthesis pathways—whether mevalonate or MEP-based—can trigger a wide spectrum of human diseases. Below, we highlight four clinically significant axes: mitochondrial disorders, glycosylation defects, cancer and immune modulation, and infectious disease drug targets, each linked to specific isoprenoid-dependent metabolites or enzymes.
Mitochondrial Disorders and CoQ Deficiency: Impact on Energy and Oxidative Stress
Coenzyme Q₁₀ (ubiquinone), derived from the mevalonate pathway, is essential for mitochondrial electron transport and redox balance. Mutations in COQ genes impair CoQ biosynthesis, causing primary CoQ deficiencies marked by myopathy, encephalopathy, and nephrotic syndrome. Unlike most mitochondrial diseases, these conditions are often responsive to high-dose CoQ supplementation, underscoring the value of early diagnosis.
Secondary CoQ deficiency may also occur in statin-treated patients, as statins suppress upstream HMG-CoA reductase activity. Reduced CoQ impairs ATP production and antioxidant capacity. LC–MS/MS quantification of ubiquinone/ubiquinol levels provides a sensitive readout of mitochondrial redox status, informing therapy in metabolic and neurodegenerative contexts.
Congenital Disorders of Glycosylation and the Dolichol Phosphate Pathway
Dolichol phosphate, a long-chain polyisoprenoid lipid, is essential for N-glycan assembly in the ER. Mutations in genes like DHDDS, NUS1, and SRD5A3 disrupt dolichol synthesis, leading to CDG type I subtypes with neurological, ophthalmic, and systemic features.
A major 2024 discovery revealed DHRSX as a previously unrecognized enzyme mediating two critical steps in polyprenol-to-dolichol conversion. This update reshapes the dolichol biosynthesis pathway, refining diagnosis and expanding the spectrum of CDG-associated mutations. Targeted lipidomics and transferrin glycoform profiling now aid in identifying dolichol-linked CDG patients. Though therapeutic options remain limited, early recognition enables supportive care and targeted trials.
Protein Prenylation in Cancer and Immune Regulation
The mevalonate pathway supplies farnesyl- and geranylgeranyl-pyrophosphate for protein prenylation, a lipid modification essential for membrane localization of Ras, Rho, and Rab GTPases. These signaling proteins regulate growth, motility, and immunity. In cancer, hyperactive Ras relies on prenylation for oncogenic function, making it a therapeutic vulnerability.
Clinically, statins—originally developed as cholesterol-lowering agents—indirectly affect prenylation by inhibiting upstream HMG-CoA reductase, depleting FPP and GGPP pools. Discovered from Penicillium mold in the 1970s, the first statin (compactin) evolved into a global class of drugs that revolutionized cardiovascular prevention. Beyond lipids, statins revealed how inhibiting isoprenoid biosynthesis alters CoQ₁₀ levels and Ras prenylation, linking lipid metabolism to cancer and mitochondrial side effects.
Farnesyltransferase inhibitors (FTIs) like lonafarnib are approved for progeria, and GGTase inhibitors are being explored for immunomodulation and tumor microenvironment reprogramming. As prenylation dynamics intersect with ferroptosis, immunity, and signaling plasticity, they offer expanding therapeutic opportunities rooted in isoprenoid control.
Infectious Disease Targets: MEP Pathway and DXR Inhibitors in Malaria and TB
The MEP pathway, absent in humans but essential in most bacteria and parasites, offers attractive targets for antimicrobial therapy. The enzyme DXR (IspC) is inhibited by fosmidomycin, which has demonstrated efficacy in Plasmodium falciparum, especially in combination regimens.
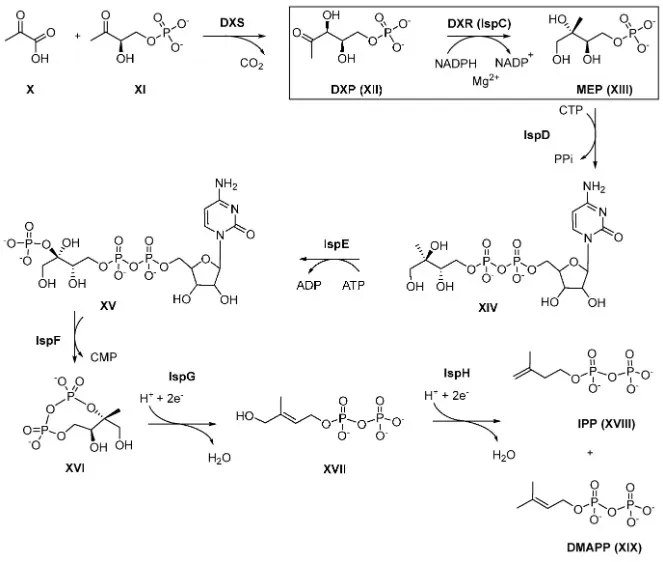
figure mep pathway dxr target knak 2022
Source: Knak T, Abdullaziz MA, Höfmann S, et al. “Over 40 years of fosmidomycin drug research,” Pharmaceuticals (2022) 15(12):1553. Figure 4 (MEP pathway and targets). Licensed under CC BY 4.0. DOI: 10.3390/ph15121553. No endorsement implied.
A landmark example is the isoprenoid-derived antimalarial artemisinin, a sesquiterpene lactone isolated from Artemisia annua by Tu Youyou, who was awarded the 2015 Nobel Prize. Artemisinin’s rapid parasite-killing activity transformed malaria treatment. Artemisinin biosynthesis in Artemisia annua primarily draws on the cytosolic mevalonate (MVA) pathway to supply farnesyl diphosphate (FPP), while metabolic crosstalk enables plastidial MEP-derived IPP/DMAPP to contribute to the sesquiterpene flux. This dual-source arrangement underlies many engineering strategies that boost artemisinin by tuning both MVA enzymes (e.g., HMGR) and key MEP nodes (e.g., DXR), as well as downstream ADS/CYP71AV1 steps.
Beyond malaria, MEP enzymes like IspD–IspH are being explored for TB and enteric pathogens. Intermediates such as HMBPP also stimulate Vγ9Vδ2 T cells, creating dual antimicrobial and immunomodulatory effects. In parallel, GGTase inhibitors are under study for pathogens like Leishmania and Trypanosoma, further extending the reach of isoprenoid-targeted anti-infectives.
Role of Isoprenoids in Plants: Light Harvesting, Structure, and Defense
Plants produce diverse isoprenoids via compartmentalized MVA (cytosol) and MEP (plastid) pathways. Chloroplast-derived carotenoids like β-carotene and lutein protect against photodamage and serve as hormone precursors. In the cytosol, phytosterols stabilize membranes and feed into brassinosteroid biosynthesis. Volatile monoterpenes and sesquiterpenes mediate herbivore defense and pollinator attraction.
A breakthrough application of plant isoprenoid engineering is Golden Rice. To combat vitamin A deficiency, researchers inserted carotenoid biosynthesis genes into rice endosperm, enabling β-carotene (a tetraterpenoid) production via the MEP pathway. This metabolic redesign transformed rice into a nutritional intervention tool, exemplifying how isoprenoid pathway engineering can address global health challenges through crop biofortification.
Table 3: Major Classes of Plant Isoprenoids and Their Functions
|
Isoprenoid Type |
Pathway Source |
Examples |
Function |
|
Carotenoids |
MEP (Plastid) |
β-Carotene, Lutein |
Light harvesting, antioxidant, hormones |
|
Phytosterols |
MVA (Cytosol) |
Sitosterol, Campesterol |
Membrane structure, hormone precursors |
|
Terpenoid volatiles |
MEP / MVA |
Limonene, Pinene |
Herbivore defense, signaling, aroma |
|
Isoprene (C₅) |
MEP (Plastid) |
Isoprene gas |
Thermal stress response in leaves |
This biochemical versatility makes carotenoid biosynthesis in plants and broader isoprenoid metabolism essential to plant survival and offers engineering targets for crop improvement.
Applications in Daily Life: From Nutrition to Synthetic Biology
Isoprenoids are deeply embedded in daily life through nutrition, fragrance, and biomanufacturing. Popular dietary supplements such as CoQ₁₀ and β-carotene support mitochondrial health and provide provitamin A, respectively. Many essential oils (e.g., linalool in lavender, limonene in citrus) derive their scent and bioactivity from isoprenoids.
In industry, engineered microbes such as yeast and microalgae are increasingly used to produce high-value isoprenoids—including nootkatone, artemisinin precursors, and biofuels like β-farnesene. These advances in yeast isoprenoids and microalgae isoprenoid production enable scalable, sustainable alternatives to plant extraction.
As synthetic biology evolves, isoprenoid pathways continue to serve as flexible platforms for producing fragrances, nutraceuticals, and next-generation biochemicals—linking metabolic fundamentals to real-world innovation.
Isoprenoids FAQ
1. What are isoprenoids used for in cells?
Isoprenoids serve as membrane components (cholesterol, phytosterols), electron carriers (CoQ₁₀), glycosylation anchors (dolichol), signaling lipids (prenyl groups), pigments (carotenoids), and hormones (gibberellins, brassinosteroids).
2. How do the mevalonate (MVA) and MEP pathways differ?
The MVA pathway (cytosolic, in animals/fungi/plants) uses acetyl-CoA; the MEP pathway (plastidic or bacterial) uses pyruvate and glyceraldehyde-3-phosphate. Both yield IPP/DMAPP but involve distinct enzymes and compartments.
3. Why is the MEP pathway a good antibiotic target?
Humans lack the MEP pathway. Inhibiting enzymes like DXR (e.g., with fosmidomycin) selectively kills pathogens such as Plasmodium falciparum and Mycobacterium tuberculosis, making it ideal for antimicrobial drug development.
4. What changed in the 2024 dolichol biosynthesis pathway update?
New evidence showed that DHRSX, not just SRD5A3, is essential for converting polyprenol to dolichol. This revised pathway clarifies the genetic basis of certain CDG subtypes and improves diagnostic accuracy.
5. How is CoQ or dolichol quantified in labs?
Using LC–MS/MS with isotope-labeled internal standards. CoQ’s redox forms (ubiquinone/ubiquinol) are measured in plasma or tissue; dolichol profiling requires lipidomics methods with careful sample handling.
6. Do statins affect prenylation or CoQ levels?
Yes. By inhibiting HMG-CoA reductase, statins reduce FPP and GGPP, which may impair protein prenylation and lower CoQ₁₀ levels—potentially contributing to muscle side effects in some users.
7. Can metabolic engineering produce isoprenoids at scale?
Yes. Engineered microbes like yeast and microalgae now produce β-farnesene, artemisinin precursors, and nootkatone. Challenges include metabolic burden, product toxicity, and enzyme expression—but progress is rapid.
Unlocking Isoprenoid Biology with Targeted Metabolomics and Lipidomics
Isoprenoids shape core cellular processes—from mitochondrial energy production to glycoprotein assembly and small GTPase signaling. Disruptions in their biosynthesis impact metabolism, immunity, and disease. With expanding roles in health and biotechnology, understanding isoprenoid pathways is essential for modern life science research.
MetwareBio offers targeted metabolomics and lipidomics to support precise analysis of CoQ₁₀, dolichol, FPP/GGPP, and more.
Reference
- Staiano C, García-Corzo L, Mantle D, et al. Biosynthesis, deficiency, and supplementation of coenzyme Q. Antioxidants. 2023;12(7):1469. doi:10.3390/antiox12071469
- Wang Y, Lilienfeldt N, Hekimi S. Understanding coenzyme Q. Physiol Rev. 2024;104(4):1533-1610. doi:10.1152/physrev.00040.2023.
- Jain A, Li VW, Salem J, Reddy ST, Palaskas NJ, Meriwether D. A novel application of LC–MS/MS accurately quantifies the labile redox pools of cellular coenzymes Q9 and Q10. Free Radic Biol Med. 2025;240:96-107 (ahead of print). doi:10.1016/j.freeradbiomed.2025.08.022.
- Wilson MP, Kentache T, Althoff CR, et al. A pseudoautosomal glycosylation disorder prompts the revision of dolichol biosynthesis. Cell. 2024;187(14):3585–3601.e22. doi: 10.1016/j.cell.2024.04.041
- Kentache T, Althoff CR, Caligiore F, et al. Absence of the dolichol synthesis gene DHRSX leads to N-glycosylation defects in Lec5 and Lec9 Chinese hamster ovary cells. J Biol Chem. 2024;300(12):107875. doi:10.1016/j.jbc.2024.107875
- Chang IJ, He M, Lam CT. Congenital disorders of glycosylation. Ann Transl Med. 2018;6(24):477. doi:10.21037/atm.2018.10.45
- Mattingly JR, Wu A, York AG. Regulation of adaptive immunity by lipid post-translational modifications. Immune Netw. 2025;25(1):e11. doi:10.4110/in.2025.25.e11
- Yuan Y, Li P, Li J, Zhao Q, Chang Y, He X. Protein lipidation in health and disease: molecular basis, physiological function and pathological implication. Signal Transduct Target Ther. 2024;9:60. doi:10.1038/s41392-024-01759-7.
- Budzinska A, Jarmuszkiewicz W. The cellular and mitochondrial consequences of mevalonate pathway inhibition by nitrogen-containing bisphosphonates: a narrative review. Pharmaceuticals (Basel). 2025;18(7):1029. doi:10.3390/ph18071029
- Pu X, Dong X, Li Q, Chen Z, Liu L. An update on the function and regulation of MEP and MVA pathways and their evolutionary dynamics. J Integr Plant Biol. 2021;63(7):1211-1226. doi:10.1111/jipb.13076.
- Knak T, Abdullaziz MA, Höfmann S, et al. Over 40 years of fosmidomycin drug research: a comprehensive review and future opportunities. Pharmaceuticals (Basel). 2022;15(12):1553. doi:10.3390/ph15121553
- Tetali SD. Terpenes and isoprenoids: a wealth of compounds for global use. Planta. 2019;249(1):1–8. doi:10.1007/s00425-018-3056-x
- Palmer AC. Golden Rice: A Quarter-Century of Innovation, Challenges, and the Promise of Better Nutrition. J Nutr. Published online July 2, 2025. doi:10.1016/j.tjnut.2025.06.025
- Zhao L, Zhu Y, Feng W, et al. From plant to yeast—advances in biosynthesis of artemisinin. Molecules. 2022;27(20):6888. doi:10.3390/molecules27206888
- Mohamadnia S, Valverde-Pérez B, Tavakoli O, et al. Progress and prospects in metabolic engineering approaches for isoprenoid biosynthesis in microalgae. Biotechnol Biofuels Bioprod. 2025;18:64. https://doi.org/10.1186/s13068-025-02665-y
Read more
- LC-MS VS GC-MS: What's the Difference
- LC vs. HPLC vs. UHPLC: Tracing the Evolution of Chromatographic Techniques
- Mastering Chromatography: Everything You Need to Know
- DIA Proteomics vs DDA Proteomics: A Comprehensive Comparison
- Multi-Omics Association Analysis Series
- Omics Data Processing Series
- Omics Data Analysis Series
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


