Mass Spectrometry Acquisition Mode Showdown: DDA vs. DIA vs. MRM vs. PRM
Mass spectrometry (MS) is a powerful analytical technique that has revolutionized research across proteomics, metabolomics, pharmaceutical sciences, and clinical diagnostics. Its strength lies in its ability to accurately detect, identify, and quantify a wide array of biomolecules—even at trace levels. However, the data acquisition strategy chosen in an MS workflow plays a critical role in determining the depth, sensitivity, and reproducibility of results.
This guide offers an in-depth comparison of the four most commonly used MS acquisition modes: Data-Dependent Acquisition (DDA), Data-Independent Acquisition (DIA), Multiple Reaction Monitoring (MRM), and Parallel Reaction Monitoring (PRM). From the architecture of a mass spectrometer and the principles of compound identification to the detailed mechanisms of different scan modes, this guide will walk you through the similarities and differences among the four acquisition strategies—helping you make an informed decision when selecting the method that best fits your analytical goals.
Structural and Functional Foundations of Mass Spectrometry
Since different acquisition modes are essentially defined by how key components of the mass spectrometer are configured and operated, it is important to first understand the instrument’s structural layout. A standard mass spectrometer consists of several core elements, each fulfilling a distinct role in the ionization, selection, fragmentation, and detection of analytes:
Ion Source
This component converts neutral molecules into charged ions using methods such as Electrospray Ionization (ESI) or Matrix-Assisted Laser Desorption/Ionization (MALDI). Ionization is a critical step that determines the efficiency and compatibility of MS analysis for various sample types.
Mass Analyzer(s)
The mass analyzer separates ions based on their mass-to-charge ratio (m/z). Depending on the instrument configuration, analyzers such as quadrupoles, time-of-flight (TOF), ion traps, or Orbitraps may be used individually or in tandem (as in MS/MS workflows) to offer varying levels of resolution and speed.
Collision Cell (Q2)
In tandem MS setups, the collision cell—positioned between two mass analyzers—plays a vital role. It introduces an inert gas (such as nitrogen or argon) that collides with isolated precursor ions, causing them to fragment in a controlled process known as collision-induced dissociation (CID) or higher-energy collisional dissociation (HCD). The resulting fragments carry important structural information for compound identification.
Detector
After ion separation and fragmentation, the detector quantifies the intensity of ions at each m/z value. These data points are translated into a mass spectrum, which serves as the analytical fingerprint of the compounds in the sample.
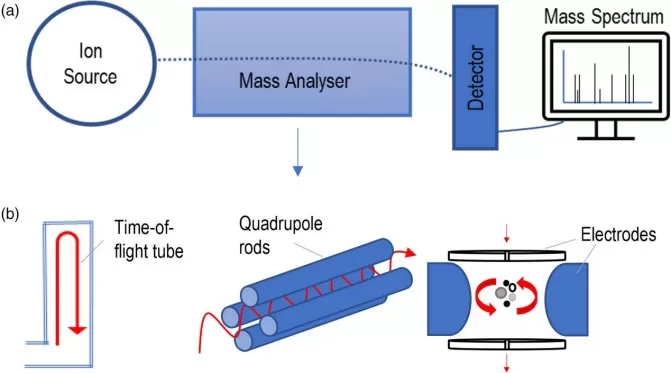
Core Components of a Mass Spectrometer and Common Types of Mass Analyzers (Keane et al., 2024)
Data Acquisition Logic in Tandem Mass Spectrometry
Following the introduction of mass spectrometer components, the next step is to understand how these elements operate together during analysis. The processes of ion selection, fragmentation, and detection are governed by specific scanning and identification strategies. These strategies form the methodological basis of the four most widely used MS data acquisition modes.
Scanning Strategies: Full Scan vs. Selected Scan
One of the most fundamental concepts in mass spectrometry is the scanning strategy used to acquire ion data. Mass analyzers can operate in two basic modes: full scan and selected scan.
- In full scan mode, the instrument records signals for all ions within a defined m/z range. This mode provides a comprehensive snapshot of the sample and is widely used in untargeted analysis, where the goal is to detect as many compounds as possible without prior knowledge.
- In contrast, selected scan mode focuses only on ions of specific interest—either known precursor ions or their corresponding fragment ions. This mode enhances sensitivity, specificity, and reproducibility, making it the strategy of choice for targeted quantification and validation studies.
These two scanning approaches are foundational to how MS data is collected and interpreted, and their combination across different acquisition modes defines both the depth and focus of an experiment.
Compound Identification: MS1 and MS2 Scans
Mass spectrometry identifies molecules using a two-stage scan process, known as tandem MS or MS/MS. This technique enables both molecular detection and structural elucidation through complementary scans:
- The MS1 scan (also called the precursor scan or survey scan) detects intact ions across a selected m/z range. This full-spectrum scan offers a global view of all ionizable species in the sample.
- The MS2 scan (also called the fragmentation scan) isolates and fragments selected precursor ions, generating unique product ion spectra. These fragmentation patterns reveal structural features that are used to identify and differentiate compounds.
By comparing the fragment spectra from MS2 to known patterns in spectral libraries or using computational predictions, researchers can confirm compound identity with high confidence. This two-tiered scan strategy is what makes mass spectrometry such a powerful tool for both discovery-based and targeted analytical workflows.
The Tandem MS Workflow
In a tandem mass spectrometry (MS/MS) system, ions follow a defined path from introduction to detection. First, ions are generated in the ion source and given a charge. These charged ions then enter the first mass analyzer (Q1), which either scans broadly (full scan) or filters specific m/z values (selected scan), depending on the acquisition strategy. The selected ions next pass through the collision cell (Q2), where they are fragmented into product ions via collision-induced dissociation (CID). These fragments then travel to the second mass analyzer (Q3), which again may perform either a full scan or selected scan to detect the resulting fragments.
The combination of scan modes used in Q1 and Q3 defines the data acquisition mode. There are four widely used acquisition strategies, each built from a different pairing of scan types:
- DDA (Data-Dependent Acquisition) and DIA (Data-Independent Acquisition) both rely on full scan strategies in Q1 and/or Q3, making them ideal for untargeted workflows that aim to profile a wide range of analytes.
- MRM (Multiple Reaction Monitoring) and PRM (Parallel Reaction Monitoring) employ selected scan techniques to focus on predefined targets, offering the highest levels of precision and sensitivity for targeted analysis.
Data-Dependent Acquisition (DDA): Principle, Strengths, and Applications
DDA is a foundational data acquisition strategy in untargeted mass spectrometry. It remains a popular approach in proteomics and metabolomics for identifying a broad range of molecular species.
DDA Working Principle: Precursor-Driven Fragmentation in Full Scan Mode
In DDA, the MS instrument first performs a full MS1 scan to detect all ions across a wide m/z range. Based on real-time intensity ranking, it automatically selects the top N most abundant precursor ions (e.g., top 10 or 20) for MS/MS analysis. The selected precursors are isolated by the first quadrupole (Q1), fragmented in the collision cell (Q2) using collision-induced dissociation (CID), and the resulting fragment ions are captured in full scan mode by the third analyzer (Q3). This precursor-dependent fragmentation makes DDA ideal for identifying unknown compounds in complex mixtures.
DDA Strengths and Limitations in Untargeted Analysis
DDA provides high-resolution, clean MS2 spectra that support confident identification of abundant molecules. However, it is inherently biased toward high-intensity ions, which can result in poor reproducibility across replicates. Low-abundance compounds are often missed due to dynamic exclusion, and quantification accuracy is generally weaker than that of targeted approaches.
DDA Applications in Untargeted Proteomics and Metabolomics
DDA is widely used in untargeted proteomics for protein identification, pathway discovery, and spectral library generation. It is also applied in untargeted metabolomics, where comprehensive compound coverage is essential. Although newer acquisition modes offer greater consistency, DDA remains a standard tool in exploratory MS-based research.
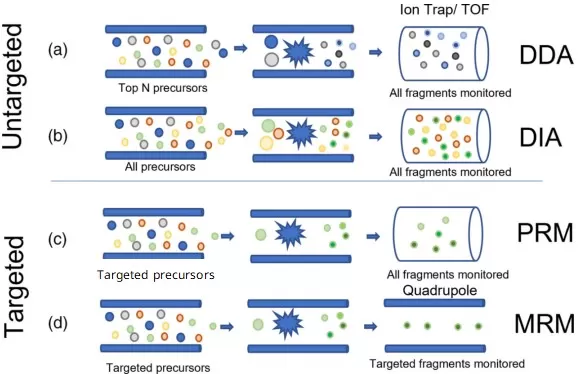
Working Principles of DDA, DIA, MRM, and PRM Acquisition Modes in Mass Spectrometry (adapted from Keane et al., 2024)
Data-Independent Acquisition (DIA): Principle, Strengths, and Applications
DIA is an untargeted acquisition technique designed to enhance reproducibility and quantitative accuracy in complex sample analysis. It has become a mainstay in large-scale, label-free proteomics.
DIA Working Principle: Wide Window Fragmentation for Full Ion Coverage
DIA divides the full m/z range into consecutive, wide isolation windows—typically 20 to 25 Da each. During acquisition, the first quadrupole (Q1) systematically selects one window at a time and transmits all ions within that range to the collision cell (Q2). These ions undergo simultaneous fragmentation, and all resulting product ions are collected by the final analyzer (Q3 or a high-resolution Orbitrap) in full scan mode. This unbiased, window-based approach allows comprehensive MS/MS data acquisition for nearly all detectable precursor ions, independent of their intensity.
DIA Strengths and Limitations in Quantitative Omics
DIA offers excellent reproducibility and is capable of detecting low-abundance analytes with high sensitivity. It supports label-free quantification, retrospective data analysis, and deep proteome coverage. However, DIA’s multiplexed MS2 spectra are complex and require advanced deconvolution algorithms and high-quality spectral libraries for accurate interpretation.
DIA Applications in Untargeted Quantitative Proteomics
DIA has become the method of choice for large-scale quantitative proteomics, especially in clinical cohort studies, longitudinal profiling, and biomarker discovery. It is also gaining traction in untargeted metabolomics, where consistency across runs is critical. The technique is ideal for studies requiring robust, reproducible, and data-rich untargeted analysis.
Multiple Reaction Monitoring (MRM): Principle, Strengths, and Applications
MRM is the most established acquisition mode for targeted quantification in mass spectrometry. It is the standard in regulated environments such as clinical diagnostics and pharmaceutical development.
MRM Working Principle: Targeted Monitoring via Predefined Transitions
MRM is executed on triple quadrupole instruments, using predefined precursor-to-product ion transitions called MRM transitions. The first quadrupole (Q1) filters the target precursor ion with a narrow m/z window. The selected ion is fragmented in the collision cell (Q2), and the third quadrupole (Q3) selectively transmits only specific fragment ions. Each transition is optimized in advance, enabling highly selective and sensitive detection of known compounds with minimal interference from background noise.
MRM Strengths and Limitations in Targeted Quantification
MRM delivers unmatched specificity, linearity, and reproducibility, making it the gold standard for targeted MS workflows. It is especially effective for low-concentration analytes in complex biological matrices. However, it is limited to known targets and requires significant upfront effort to optimize transitions, which may limit scalability for larger panels.
MRM Applications in Targeted Metabolomics and Clinical Bioanalysis
MRM is extensively applied in targeted metabolomics for the quantification of metabolic biomarkers, amino acids, lipids, and xenobiotics. It is also essential in therapeutic drug monitoring, clinical assay validation, and pharmacokinetics/pharmacodynamics (PK/PD) studies. In regulatory environments, MRM is preferred for its accuracy, compliance compatibility, and robustness.
Parallel Reaction Monitoring (PRM): Principle, Strengths, and Applications
PRM is a high-resolution targeted acquisition mode that combines the specificity of MRM with the spectral richness of full-scan MS/MS. It is particularly valuable in protein biomarker validation workflows.
PRM Working Principle: Full Fragment Ion Spectra for Targeted Ions
PRM is performed on high-resolution instruments like Orbitraps or Q-TOFs. The method begins with narrow-window isolation of a predefined precursor ion in Q1. This ion is fragmented in Q2 via CID, and all resulting product ions are captured by the high-resolution analyzer in a full MS2 scan. This parallel recording of complete fragment ion spectra enables confident identification and flexible post-acquisition quantification.
PRM Strengths and Limitations in Targeted Proteomics
PRM offers high mass accuracy, selectivity, and flexibility without the need to pre-select individual transitions. This allows users to extract the most reliable fragments retrospectively. While easier to develop than MRM, PRM may be limited in throughput due to longer scan times and is typically not suited for very large target panels.
PRM Applications in Targeted Protein Quantification
PRM is widely used in targeted proteomics, especially for verification of candidate biomarkers, phosphopeptide quantification, and post-translational modification (PTM) analysis. It is ideal for applications that require both quantitative accuracy and structural confidence. PRM is also often employed as a validation step following DIA-based discovery.
DDA vs. DIA vs. MRM vs. PRM: What’s the Difference?
Although DDA, DIA, MRM, and PRM differ significantly in their acquisition logic and analytical focus, they can all be understood as combinations of how ions are selected and how fragment data are acquired. DDA and DIA are primarily used for untargeted analysis, while MRM and PRM are optimized for targeted quantification. Additionally, DDA and MRM rely on predefined ion selection, whereas DIA and PRM capture broader or full-spectrum fragment data.
To better understand their technical differences and appropriate use cases, the table below summarizes these four acquisition modes across key parameters such as scan strategy, quantification accuracy, spectral complexity, and typical applications.
Comparison of DDA, DIA, MRM, and PRM Acquisition Modes in Mass Spectrometry
|
Feature |
DDA |
DIA |
MRM |
PRM |
|
Analysis Type |
Untargeted |
Untargeted |
Targeted |
Targeted |
|
Precursor Selection |
Real-time (top N) |
All ions in defined windows |
Predefined |
Predefined |
|
Fragment Ion Detection |
Full MS2 scan |
Full MS2 scan |
Selected transitions |
Full MS2 scan |
|
Instrument Type |
Q-TOF, Orbitrap |
Q-TOF, Orbitrap |
Triple Quadrupole |
Orbitrap, Q-Exactive |
|
Spectral Complexity |
Low |
High |
Low |
Moderate |
|
Quantification Precision |
Moderate |
High |
Very High |
High |
|
Sensitivity |
Moderate |
High |
Very High |
High |
|
Reproducibility |
Low–Moderate |
High |
Very High |
High |
|
Throughput |
High |
Medium |
High |
Medium |
|
Library Requirement |
Optional |
Recommended |
Not needed |
Optional |
|
Best For |
Discovery & ID |
Quantitative omics |
Clinical quantification |
Confident targeted validation |
Reference
Keane, R. E., Tidy, R. J., Parker, G. J., Gummer, J. P. A., & Priddis, C. (2024). Mass spectrometry based proteomics: Changing the impact of protein analysis in forensic science. WIREs Forensic Science, 6(4), e1516. https://doi.org/10.1002/wfs2.1516
Bezstarosti, K., Van der Wal, L., Doff, W. A. S., & Demmers, J. A. A. (2024). Parallel reaction monitoring targeted mass spectrometry as a fast and sensitive alternative to antibody-based protein detection. Frontiers in Analytical Science, 4. https://doi.org/10.3389/frans.2024.1397810
Lan, Y., Zeng, X., Xiao, J., Hu, L., Tan, L., Liang, M., Wang, X., Lu, S., Long, F., & Peng, T. (2021). New advances in quantitative proteomics research and current applications in asthma. Expert review of proteomics, 18(12), 1045–1057. https://doi.org/10.1080/14789450.2021.2017777
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


