Microfluidic Proteomics: The 'Chip Lever' Unlocking Low-Input Proteomics
Microfluidic proteomics refers to lab-on-a-chip approaches for protein analysis, where sample preparation, separation, reactions, and enrichment are miniaturized and integrated onto a device about the size of a postage stamp. By precisely manipulating fluids in the picoliter to nanoliter range, these platforms enable “fine-grained cultivation” of tiny samples that would otherwise be impossible to analyze. This technology is designed to solve a core challenge in proteomics: how to identify and quantify thousands of proteins when starting material is extremely limited (for example, a single cell or a minute tissue biopsy). By shrinking the reaction volumes and surfaces, microfluidic proteomics drastically reduces sample losses and increases sensitivity, making low-input and single-cell proteomics feasible. Notably, recent microfluidic methods have demonstrated the ability to quantify on the order of 3,000+ proteins from a single human cell [1]. Microfluidic proteomics is transforming proteomics by bringing the entire workflow onto tiny chip-based devices, unlocking analyses of samples at scales (single cells, sub-microgram inputs) that were once out of reach.
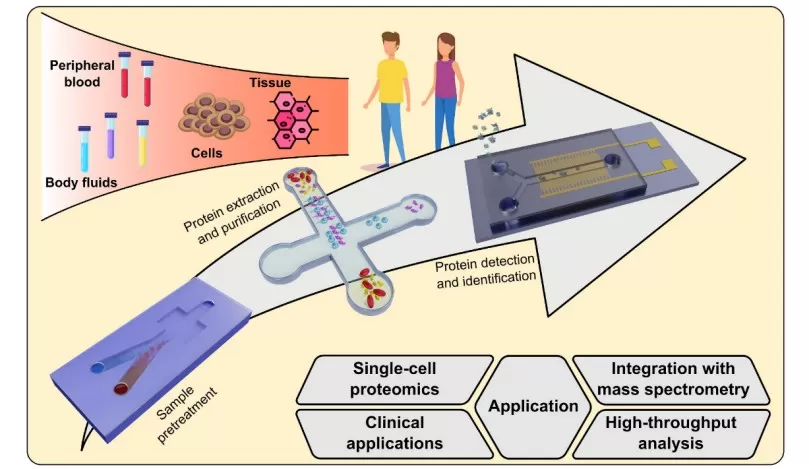
Microfluidics-Based Proteomics
Core Technologies: How Does "On-Chip Enrichment" Work?
Achieving “on-chip enrichment” in proteomics involves a toolbox of microfluidic techniques. Rather than a single method, microfluidic enrichment encompasses multiple core strategies that concentrate and clean up proteins/peptides in tiny volumes. Key technologies include:
1. Micro Solid-Phase Extraction (μSPE): Tiny solid-phase extraction columns or micropillar arrays are integrated into microfluidic channels to capture target proteins or peptides. The sample is flowed through these micro-columns where analytes bind to a stationary phase (e.g. reversed-phase or ion-exchange material). Unwanted salts and contaminants are then washed away, and the analytes are eluted in very small volumes, yielding a highly concentrated sample plug. The μSPE approach essentially functions as an on-chip microextraction and pre-concentration module, improving recovery and detection of low-abundance peptides. Because the columns are microfabricated in-place, fluidic connections are minimal and dead volumes are nearly eliminated. (In fact, a commercial example of this concept is the cHiPLC® system, which docks microfluidic nanoLC/trap column chips with negligible dead volume and excellent reproducibility)
2. Nanodroplet Processing: Instead of channels and columns, another paradigm is to partition the sample into nanoliter-sized droplets that act as isolated microreactors. Each droplet (often on an open chip or slide surface) contains a micro-sample and all necessary reagents for lysis, digestion, and labeling. Every droplet is effectively a miniature test tube, but without the large walls – this dramatically reduces surface adsorption losses. The nPOP method exemplifies this: single cells are dispensed into 10–20 nL droplets on a specially coated glass slide, and all subsequent reactions (protein extraction, trypsin digestion, chemical labeling) occur within those droplets. By confining reactions to such ultra-small volumes, nanodroplet processing achieves high local concentrations of enzymes and labeling reagents, speeding up reactions and improving yield while using minimal reagent. Another benefit is that because volumes are so small, the total amount of reagents needed per sample is tiny – nPOP reports a reagent cost of only about $0.12 per cell processed, illustrating the cost-efficiency of this scaled-down chemistry.
3. Electrokinetic Enrichment: Microfluidic chips can also harness electric fields to concentrate proteins or peptides. Techniques such as isotachophoresis or electric field gradient focusing use a voltage applied across microchannels to mobilize and focus molecules into a tight zone based on their charge or size. For example, at a junction or a nanoporous membrane in the channel, target molecules can be trapped and concentrated by the electric field, while impurities either move out of the zone or remain diluted. This electrokinetic approach can achieve enrichment factors of several orders of magnitude within minutes, without any added reagents – essentially “powering up” the tiny sample by collecting it into a sharp band. Such methods are particularly useful for on-chip desalting or focusing of peptides before injection into a mass spectrometer [2].
4. Integrated NanoLC-Chip Systems: This approach combines enrichment and separation on the same microfluidic device. A prime example is an integrated chip that contains both a μSPE trap column and a nanoflow LC analytical column etched or packed into the chip. After on-chip enrichment, the trapped peptides can be directly eluted through the analytical column (which is also part of the chip) and into the mass spectrometer. Chip-based columns can provide reliable, consistent nano-flow separations with the convenience of quick chip swaps, all while preserving or improving chromatographic performance. In modern microfluidic proteomics, integrated chip systems continue to evolve – including devices that perform digestion, peptide cleanup, and nanoLC separation in one platform [3].
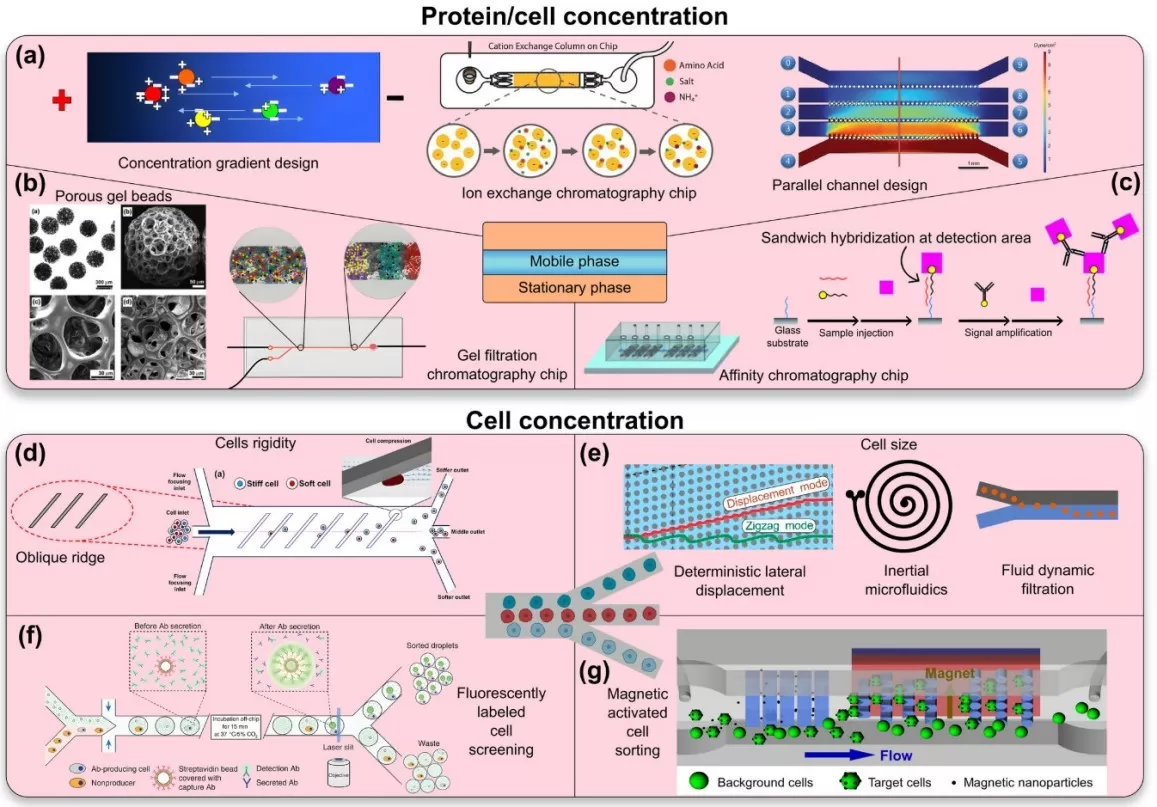
Microfluidic Protein/Cell Concentration Chip Schematic
Why Microfluidics? Irreplaceable Advantages
Microfluidic proteomics offers a set of irreplaceable advantages over conventional bench-top methods, especially when dealing with precious, low-abundance samples. Some of the key benefits include:
- Ultra-Low Sample Loss: By performing the entire sample prep within a closed microchip or droplet, microfluidic workflows avoid transfers between tubes and vials that would otherwise lead to significant losses. The sample essentially stays “locked” on the chip from start to finish. This is critical for single-cell and low-input proteomics, where every molecule counts. Microfluidic methods can capture a far greater fraction of the sample’s proteome than traditional methods, which often lose the majority of peptides during cleanup.
- High Reaction Efficiency and Speed: Working at the microscale not only saves sample, it also accelerates biochemical reactions. In tiny volumes, diffusion distances are short and reagents quickly reach optimal concentrations. Enzymatic reactions (such as trypsin digestion) and chemical labeling can proceed faster and more completely when confined to nanoliter droplets or microchambers.
- Automation & Massive Parallelization: Microfluidics readily lends itself to automation and high-throughput processing. Once a chip design or droplet pattern is established, the process can be repeated in parallel for dozens or even hundreds of samples simultaneously. For example, a single microfluidic chip may incorporate an array of 8, 16, or 96 microreactors operating in parallel.
- Seamless Integration with MS (Minimal Dead Volume): Microfluidic enrichment devices can be coupled almost directly to the mass spectrometer, reducing dead volumes and transition losses to nearly zero. Microfluidics provides a near-frictionless link between sample prep and MS analysis, which translates to better peptide separation, higher signal intensities, and deeper proteome coverage of complex biological samples.
Revolutionary Applications: From Single Cells to Clinics
The advent of microfluidic enrichment techniques has catalyzed revolutionary applications in proteomics, enabling studies that were previously impractical or unimaginable. A few prominent examples include:
Single-Cell Proteomics at Scale: Perhaps the most paradigm-shifting impact of microfluidic proteomics is in single-cell mass spectrometry (MS) proteomics, typified by workflows like SCoPE-MS/SCoPE2. Microfluidic handling is the linchpin that allows hundreds to thousands of individual cells to be processed in parallel with reproducibility. By combining on-chip sample prep with multiplexed isobaric tagging, SCoPE2 (using 32-plex TMT labels) has quantified on the order of 1,000+ proteins per single mammalian cell, analyzing over 1,000 single cells per day in MS runs.
Rare or Tiny Clinical Samples: Microfluidic proteomics is also breaking barriers in clinical and translational research, by enabling deep proteome analysis of samples that are extremely limited in quantity. For example, fine-needle biopsy samples, circulating tumor cells (CTCs) isolated from blood, or even extracellular vesicles (exosomes) typically contain only picogram to nanogram levels of protein, which used to be insufficient for standard proteomic workflows. With microfluidic enrichment, these rare samples can be processed with minimal loss and high sensitivity. Researchers can now profile the proteome of a handful of cells from a patient biopsy or a few CTCs to identify biomarkers, whereas traditional methods needed millions of cells. The ability to get meaningful proteomic data out of minuscule clinical specimens is something only microfluidic-based workflows are capable of at the moment, highlighting their irreplaceable value in biomedical applications.
Spatial and Microdissected Proteomics: Another frontier opened by microfluidic sample processing is spatially-resolved proteomics – analyzing proteins in specific microscopic regions of a tissue. This integration of microfluidics with spatial biology tools effectively creates a bridge between histology and proteomics. This merging of proteomics with spatial resolution stands to significantly advance fields like pathology and developmental biology, where understanding the local protein context is crucial.
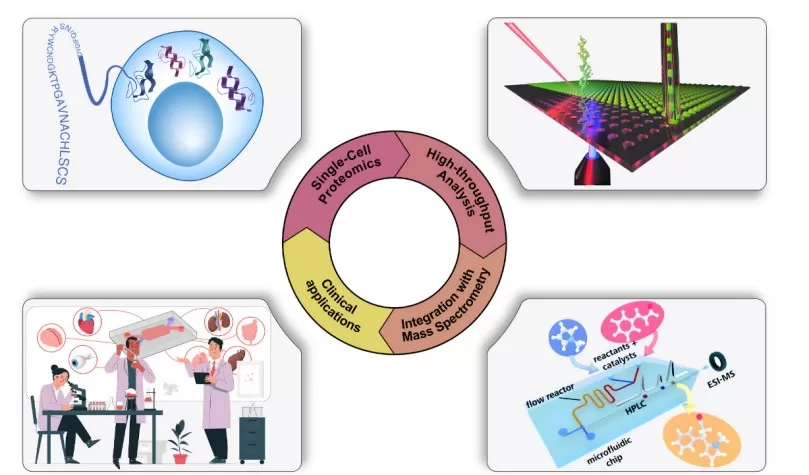
Applications of Microfluidics in Proteomics
Integration with LC-MS/MS Platforms
For microfluidic proteomics to be truly impactful, it must interface smoothly with the analytical engine of proteomics: liquid chromatography-tandem mass spectrometry (LC-MS/MS). A major strength of microfluidic systems is their seamless integration with LC-MS platforms, achieved through clever engineering that minimizes any gap between on-chip processing and MS injection. One approach to integration is via chip-based nanoLC. In this case, the microfluidic device itself includes the chromatography component, and it docks directly into the mass spec. The classic example is the Agilent HPLC-Chip, which had a microfabricated capillary column on a chip plus an electrospray emitter – the whole chip would plug into a port on the mass spectrometer. Another approach is the open-slide or digital microfluidic integration, used by methods like nPOP. Here, the sample preparation happens on an open surface (slides or a digital microfluidic device), and after processing, the samples are gathered and introduced to a standard LC-MS system. The integration is achieved by designing the collection format to be MS-friendly.
Challenges and Future Directions
Despite its promise and demonstrated successes, microfluidic proteomics still faces several challenges that must be addressed as the field moves forward. Likewise, there are exciting future directions aimed at overcoming these hurdles and expanding the technology’s capabilities.
Current Challenges:
Device Fabrication and Cost: High-performance microfluidic chips (especially those made of glass, silicon, or advanced polymers) can be expensive and complex to fabricate.
Standardization and Reproducibility: Many microfluidic proteomics workflows have been developed in individual labs and are not yet standardized or commercialized to the extent of traditional kits. The field is working toward community guidelines and cross-lab benchmarking (for example, the nPOP developers have made their protocol public and even provided an R package, QuantQC, for quality control).
Technical Expertise and Training: Operating microfluidic devices and troubleshooting them can require a specialized skill set. These systems often demand careful handling of tiny volumes, awareness of fluidic phenomena like evaporation or channel clogging, and proficiency with associated software /hardware.
Future Directions:
Lower-Cost, Scalable Manufacturing: A clear direction is to develop microfluidic chips that are cheaper and easier to mass-produce. This might involve switching from glass/silicon to polymers or plastics that can be injection molded in large quantities. There is active research in 3D-printing microfluidic devices as well, which could allow rapid prototyping and dissemination of designs without expensive infrastructure.
Integration of AI and Smart Automation: As microfluidic proteomics workflows generate more data (both in terms of proteomic output and instrument log files), there is an opportunity to use artificial intelligence (AI) and machine learning to optimize processes.
Multi-Omics on Chip: Another exciting direction is extending microfluidic platforms to handle multiple types of biomolecules simultaneously, enabling multi-omics from the same low-input sample. Achieving true proteins + metabolites + transcripts analysis on one microfluidic platform would be revolutionary – it would provide a comprehensive molecular profile of extremely limited samples or single cells, all obtained in a coordinated manner.
Conclusion
Microfluidic technology is proving to be a genuine game-changer in proteomics, providing a lever on a chip that pries open the door to analyses of extremely low-input samples. By virtue of its precision, efficiency, and low-loss processing, microfluidic proteomics is elevating the sensitivity and scale of protein analysis to unprecedented levels. But this advancement is more than just a miniaturization of existing methods; it represents a paradigm shift in how we approach biochemical analysis. We are adopting a mindset where smaller is better: smaller volumes, smaller samples, and smaller devices, all leading to bigger insights.
The implications of this shift are profound. Microfluidic proteomics is enabling us to explore the molecular intricacies of life in finer detail – be it the unique proteomic signature of an individual cell, the subtle differences in a tiny tumor biopsy, or the dynamic protein changes in a microscopic region of tissue. As the technology continues to mature, we can expect it to become more accessible and even more powerful, integrating with other omics and scaling to new heights. The “chip lever” of microfluidics is empowering scientists to ask and answer questions that were previously out of reach, driving forward our understanding of biology and disease.
References
[1] Leduc A, Khoury L, Cantlon J, Khan S, Slavov N. Massively parallel sample preparation for multiplexed single-cell proteomics using nPOP. Nat Protoc. 2024 Dec;19(12):3750-3776.
[2] Yang Z, Jin K, Chen Y, Liu Q, Chen H, Hu S, Wang Y, Pan Z, Feng F, Shi M, Xie H, Ma H, Zhou H. AM-DMF-SCP: Integrated Single-Cell Proteomics Analysis on an Active Matrix Digital Microfluidic Chip. JACS Au. 2024 Mar 26;4(5):1811-1823.
[3] Gebreyesus ST, Siyal AA, Kitata RB, Chen ES, Enkhbayar B, Angata T, Lin KI, Chen YJ, Tu HL. Streamlined single-cell proteomics by an integrated microfluidic chip and data-independent acquisition mass spectrometry. Nat Commun. 2022 Jan 10;13(1):37.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


