N-Linked vs. O-Linked Glycosylation: Structure, Function, Disease Links & Analysis
Protein glycosylation—the covalent attachment of carbohydrate chains to proteins—is one of the most pervasive and functionally diverse post-translational modifications in eukaryotes. Among its various forms, N-linked and O-linked glycosylation stand out for their critical roles in protein folding, stability, cellular recognition, and signaling. In this comprehensive guide, we delve into the structural nuances, functional implications, analytical challenges, and disease associations of these two glycosylation types, providing you with both foundational knowledge and cutting-edge insights.
What Is N-Linked Glycosylation and O-Linked Glycosylation?
N-Linked Glycosylation involves the en bloc transfer of a preassembled oligosaccharide onto the amide nitrogen of an Asn within the consensus sequence Asn–X–Ser/Thr (where X ≠ Pro) during nascent protein synthesis in the endoplasmic reticulum (ER). O-Linked Glycosylation attaches single sugar residues directly to the hydroxyl oxygen of Ser, Thr (and less commonly Tyr or Hyp) residues. Unlike N-glycans, there is no universal consensus sequence, and O-glycan cores are highly diverse. The two major subtypes are: Mucin-type (Core 1–Core 4, etc.), initiated by GalNAc-T enzymes attaching GalNAc to Ser/Thr, forming often-branched structures in secreted and membrane mucins. O-GlcNAcylation, a dynamic, regulatory modification where a single GlcNAc is added/removed on nuclear and cytosolic proteins by OGT/OGA enzymes.
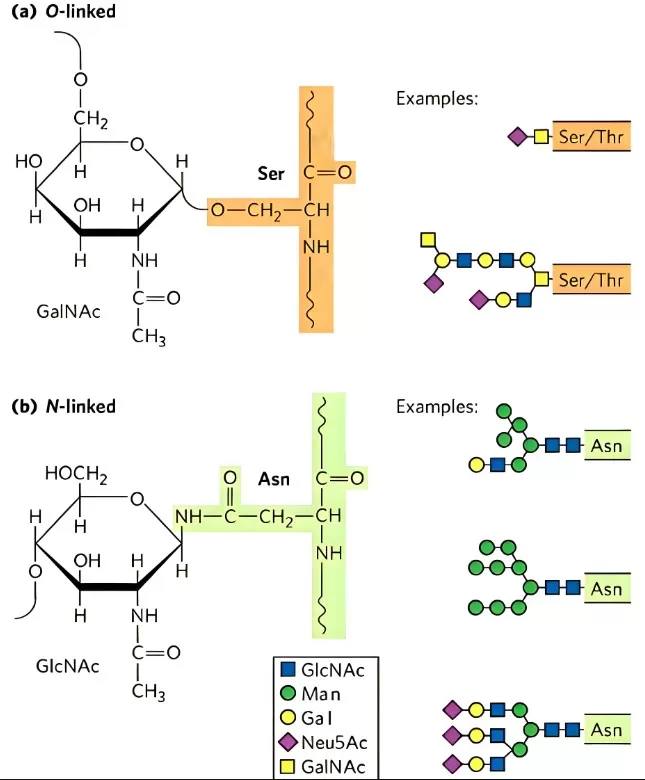
Diagram of N-Linked Glycosylation and O-Linked Glycosylation
N-Linked vs. O-Linked: Key Differences at a Glance
|
Feature |
N-Linked Glycosylation |
O-Linked Glycosylation |
|
Site & Sequence |
Asn–X–Ser/Thr (X ≠ Pro) |
No strict consensus; Ser/Thr sites |
|
Initial Core |
Man₃GlcNAc₂ (pentasaccharide) |
No universal core; multiple core types (Core 1–8, O-GlcNAc) |
|
Processing Location |
ER (assembly) → Golgi (processing) |
Golgi (extension of mucin-type); cytosol/nucleus (O-GlcNAc) |
|
Structural Diversity |
High-mannose, hybrid, complex |
Sixteen mucin cores; O-GlcNAc regulatory cycles |
|
Functional Roles |
Quality control, receptor recognition, immune modulation |
Mucin barrier formation, cell adhesion, nutrient sensing, signaling |
|
Analytical Complexity |
Glycoform heterogeneity; site-specific MS workflows needed |
Site localization challenging; O-glycan branching complexity |
Core Differences: A Deep Dive
1) Structural Features of N-Glycans and O-Glycans
N-Glycan Core & Branching
The invariant Man₃GlcNAc₂ core is elaborated by glycosidases and glycosyltransferases to yield:
- High-mannose: retention of multiple mannose residues.
- Hybrid: mannose-rich on one arm, complex sugars on the other.
- Complex: antennae of GlcNAc, Gal, and sialic acid with fucosylation variants.
These branches modulate receptor binding (e.g., selectin ligands), ER quality-control cycles via calnexin/calreticulin, and immune effector functions (e.g., Fc-glycan impact on ADCC).
O-Glycan Core Diversity
Mucin-type O-glycans initiate with GalNAc and extend into Core 1–4 structures, prevalent in secreted mucins that form protective gels on epithelial surfaces. In contrast, O-GlcNAcylation adds single GlcNAc units in a dynamic cycle akin to phosphorylation, regulating transcription factors, cytoskeletal proteins, and metabolic enzymes.
2) Functional Roles in Folding, Signaling & Immunity
N-Linked Glycosylation
- Protein Folding & Quality Control: Early trimming and reglucosylation cycles in the ER ensure only properly folded glycoproteins proceed to the Golgi.
- Cell–Cell Recognition: Specific N-glycan epitopes guide leukocyte trafficking via selectins and lectin receptors.
- Immune Modulation: Perturbations in Fc-glycan composition directly affect antibody-dependent cellular cytotoxicity (ADCC) and complement activation.
O-Linked Glycosylation
- Mucin Barrier Function: Dense O-glycan arrays on mucins provide hydration, lubrication, and pathogen entrapment in mucus layers.
- Signal Regulation: O-GlcNAc cycling acts as a nutrient sensor, modulating transcriptional networks in response to glucose levels and stress.
Analytical Strategies for Glycosylation Profiling
|
Challenge |
N-Linked Strategies |
O-Linked Strategies |
|
Glycoform Heterogeneity |
LC-MS/MS of glycopeptides; PNGase F release for glycan profiling |
Electron-transfer dissociation (ETD) to preserve glycan attachments; lectin enrichment |
|
Site-Specific Localization |
Enzymatic release + glycan tagging (e.g., 18O-labelling); MS3 workflows |
O-glycopeptide enrichment via HILIC; hydrazide chemistry |
|
Intact Glycoprotein Analysis |
Native MS, ion mobility spectrometry |
Limited; emerging top-down MS and microchip electrophoresis |
These approaches help dissect complex glycoforms, enabling both glycan composition and attachment site mapping with high confidence.
Detecting N-and O-linked oligosaccharides in glycoproteins
Glycosylation in Biologics, Biomarkers & Therapeutics
1) Biopharmaceuticals:
Precise control of N-glycan structures on monoclonal antibodies and therapeutic glycoproteins is critical for efficacy, half-life, and immunogenicity. Process analytics and glycoengineering ensure batch consistency and regulatory compliance.
2) Biomarker Discovery:
Aberrant glycosylation patterns—such as increased core-fucosylation in ovarian cancer or truncated O-glycans in colorectal tumors—serve as diagnostic and prognostic indicators in glycoproteomics studies.
3) Therapeutic Targets:
Inhibitors of glycosyltransferases (e.g., O-GlcNAc transferase) and lectin blockers offer novel strategies against cancer metastasis and viral entry processes.
Summary: Why Glycosylation Mapping Matters
N-linked and O-linked glycosylation, while sharing the overarching principle of sugar attachment to proteins, diverge profoundly in their structural cores, biosynthetic pathways, and functional outcomes. Their interplay orchestrates protein folding, cellular communication, and immune responses—underscoring the importance of advanced analytical techniques to unravel glycan complexity and its implications in health and disease.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


