PRM vs MRM: A Comparative Guide to Targeted Quantification in Mass Spectrometry
In the rapidly evolving landscape of mass spectrometry-based quantification, two dominant techniques have emerged for targeted analysis: Parallel Reaction Monitoring (PRM) and Multiple Reaction Monitoring (MRM), also known as Selected Reaction Monitoring (SRM). These methods are widely used across proteomics, metabolomics, clinical diagnostics, and biomarker validation, enabling scientists to detect and quantify specific molecules in highly complex biological matrices.
At first glance, both PRM and MRM share a common goal—accurate and sensitive detection of predefined targets—but their technical underpinnings, instrumentation requirements, and optimal use cases differ significantly. As targeted quantification becomes more integrated into translational research and regulated workflows, choosing the right method can profoundly impact data quality, throughput, and interpretation. This blog offers a clear and comprehensive comparison between PRM and MRM, highlighting how each technique works, where they excel, and what to consider when deciding between them.
What is PRM (Parallel Reaction Monitoring)?
Parallel Reaction Monitoring (PRM) is a high-resolution targeted MS/MS technique typically conducted on Orbitrap or Q-TOF instruments. In a PRM workflow, a specific precursor ion is isolated by a quadrupole and fragmented via high-energy collision-induced dissociation (HCD). Instead of monitoring only a few predefined fragment ions, PRM captures the entire MS/MS spectrum of all resulting fragments using a high-resolution, accurate-mass (HRAM) detector. This full-spectrum acquisition allows for retrospective data analysis, flexibility in fragment ion selection, and increased confidence in analyte identification. PRM is especially useful in cases involving low-abundance targets, post-translational modifications (PTMs), and samples with high background complexity, such as plasma or tissue lysates.
What is MRM (Multiple Reaction Monitoring)?
Multiple Reaction Monitoring (MRM), or SRM, is a well-established unit-resolution targeted quantification technique conducted on triple quadrupole (QQQ) mass spectrometers. It works by selecting a specific precursor ion in the first quadrupole (Q1), fragmenting it in the second quadrupole (q2), and monitoring specific, predefined fragment ions (transitions) in the third quadrupole (Q3). Only these selected transitions are recorded, making the technique exceptionally sensitive and efficient. MRM is widely used in clinical assays, pharmaceutical bioanalysis, and routine targeted proteomics, where high throughput, low cost per sample, and validated workflows are prioritized.
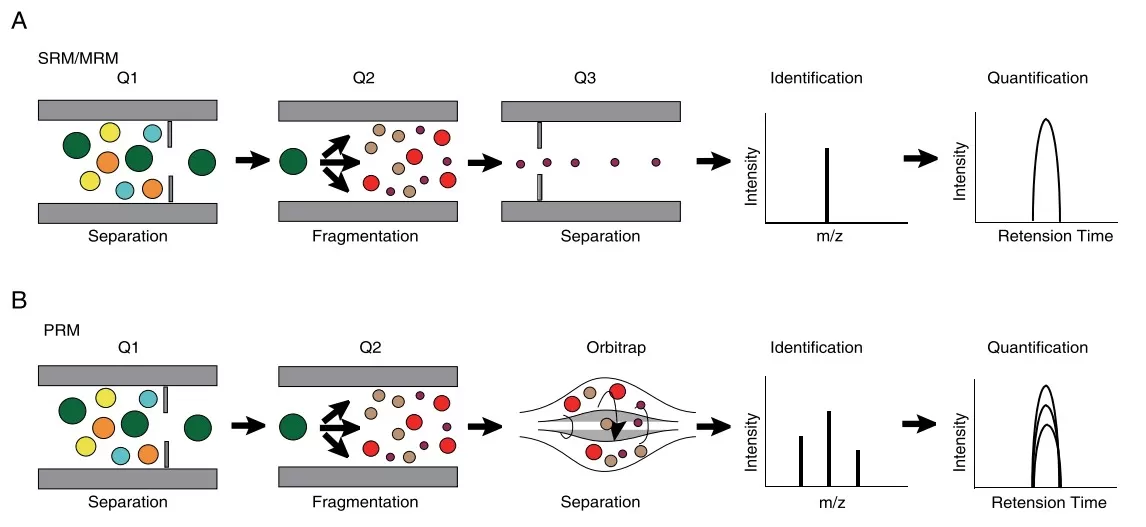
The working princeples of PRM and SRM/MRM (Lan et al., 2021)
Strengths and Weaknesses of PRM
Parallel Reaction Monitoring (PRM) offers several compelling advantages, particularly in applications that demand high selectivity and analytical flexibility. Because PRM is performed on high-resolution, accurate-mass (HRAM) instruments like Orbitrap or Q-TOF, it provides excellent mass accuracy and the ability to distinguish target ions from background interferences—even in complex biological matrices. PRM also records full MS/MS spectra, allowing researchers to extract the most suitable fragment ions post-acquisition, which enhances data confidence and supports retrospective analysis. Furthermore, PRM greatly simplifies method development by eliminating the need to pre-select and optimize fragment transitions, which is especially valuable when working with novel peptides or post-translational modifications (PTMs).
However, PRM does have limitations. The comprehensive spectral acquisition requires longer scan times per target, which can reduce the number of analytes that can be monitored in a single run—limiting its throughput in large-scale studies. Additionally, HRAM instruments are generally more expensive and require more complex maintenance and user expertise compared to triple quadrupole systems. These factors may present barriers in clinical or high-throughput labs where simplicity, speed, and cost-efficiency are critical.
Advantages and Limitations of MRM
Multiple Reaction Monitoring (MRM), performed on triple quadrupole mass spectrometers, remains the gold standard for high-throughput targeted quantification. Its greatest strength lies in sensitivity and speed: by monitoring only predefined precursor-to-fragment transitions, MRM minimizes noise and maximizes dwell time on each analyte, allowing detection of low-abundance compounds across hundreds of samples per day. MRM methods are also highly standardized and widely accepted in clinical diagnostics, pharmaceutical bioanalysis, and regulatory environments, making them a trusted choice for routine assays and long-term validated studies.
That said, MRM has some limitations. Since only a limited set of transitions are monitored, there is no opportunity for post-acquisition flexibility—if interference occurs or better transitions are later identified, the sample must be reanalyzed. Moreover, because MRM is a unit-resolution technique, it lacks the ability to resolve isobaric interferences in complex matrices, which can compromise data specificity. Method development can also be time-consuming, requiring careful optimization of collision energy, dwell time, and transition selection for each target analyte.
PRM vs SRM/MRM: What’s the Difference?
To better understand how PRM and MRM compare, the table below outlines their core differences across key technical dimensions:
Side-by-Side Comparison of PRM and MRM for Targeted MS Applications
|
Feature |
PRM |
MRM (SRM) |
|
Instrumentation |
Orbitrap, Q-TOF |
Triple Quadrupole |
|
Resolution |
High (HRAM) |
Unit resolution |
|
Fragment Ion Monitoring |
All fragments (full MS/MS spectrum) |
Predefined transitions |
|
Selectivity |
High (less interference) |
Moderate |
|
Sensitivity |
High, depending on resolution |
Very high |
|
Throughput |
Moderate |
High |
|
Method Development |
Quick, minimal optimization |
Requires transition tuning |
|
Data Reusability |
Yes (retrospective) |
No |
|
Best for |
Low-abundance targets, PTMs, validation |
High-throughput screening, routine quant |
When to Choose PRM or MRM?
Choosing between PRM and MRM depends on your analytical goals, sample complexity, and instrument availability. PRM is best suited for applications requiring high selectivity, data flexibility, and accurate quantification in complex matrices. It excels in validating low-abundance biomarkers, analyzing post-translational modifications, and supporting method development for novel targets. Because PRM captures full MS/MS spectra, researchers can revisit the data to quantify alternative fragment ions or expand target panels without re-acquiring samples—an advantage in exploratory research and evolving biomarker studies.
In contrast, MRM remains the method of choice for high-throughput, routine quantification where speed, sensitivity, and reproducibility are critical. It is ideal for clinical diagnostics, pharmacokinetics, and large-scale screening, especially when working with validated panels. MRM also offers cost-efficiency and fast analysis on triple quadrupole instruments, making it a practical solution for labs handling large sample volumes under regulated workflows. For well-characterized targets in standardized settings, MRM provides unmatched throughput and robustness.
Choose PRM When:
- You’re working with complex biological matrices where interference is a concern.
- Your targets are low-abundance or post-translationally modified peptides.
- You require retrospective data flexibility and visual confirmation of fragment co-elution.
- You’re validating new biomarkers where transition behavior is not well-characterized.
Choose MRM When:
- You need to process large sample batches quickly and cost-effectively.
- Your assay must comply with regulatory standards (e.g., clinical trials, drug testing).
- You’ve already optimized and validated your transition list.
- You’re conducting routine quantitative analysis using known targets.
Real-World Use Cases of PRM and MRM in Targeted Quantification
Targeted Proteomics
In targeted proteomics, MRM remains the preferred method in clinical and diagnostic environments due to its speed, robustness, and compatibility with validated workflows. It is commonly used to quantify protein panels in plasma, serum, and other clinical samples. In contrast, PRM is increasingly applied in exploratory research where greater specificity is needed—for example, validating signaling pathway proteins, resolving protein isoforms, or confirming multiple fragment ions for high-confidence quantification.
Clinical Metabolomics
In clinical metabolomics, MRM is widely used for quantifying metabolites in established diagnostic panels, such as amino acids, acylcarnitines, or steroid hormones. However, PRM offers distinct advantages in precision metabolic profiling, especially when distinguishing isobaric compounds or resolving background interference in complex biological matrices like urine or cerebrospinal fluid. Its high resolution and flexibility make it ideal for metabolite discovery and validation studies.
Drug Development
In the context of drug development, MRM plays a central role in pharmacokinetics (PK) and toxicokinetics (TK) due to its sensitivity, reproducibility, and ability to process large sample volumes quickly. PRM, on the other hand, is better suited for mechanistic pathway investigations, mapping protein–drug interactions, and monitoring post-translational modifications (PTMs) of therapeutic targets. Its full-spectrum acquisition is particularly useful when characterizing drug effects on biological networks at the molecular level.
PRM vs MRM as Complementary Targeted MS Tools
Parallel Reaction Monitoring (PRM) and Multiple Reaction Monitoring (MRM) are not mutually exclusive technologies but rather complementary approaches within the broader landscape of targeted mass spectrometry. MRM stands out for its high throughput, sensitivity, and standardization, making it ideal for established assays and routine clinical applications. In contrast, PRM offers greater selectivity, post-acquisition flexibility, and analytical depth, making it the method of choice for exploratory research, low-abundance targets, and complex matrices.
The optimal choice between PRM and MRM depends on your analytical priorities—whether you need speed and scalability (MRM), or specificity and adaptability (PRM). In many workflows, researchers may benefit from integrating both techniques: using MRM for screening and quantitation, and PRM for validation and in-depth analysis. Understanding the unique strengths and trade-offs of each method empowers researchers to design smarter, more effective targeted MS strategies.
Reference
Lan, Y., Zeng, X., Xiao, J., Hu, L., Tan, L., Liang, M., Wang, X., Lu, S., Long, F., & Peng, T. (2021). New advances in quantitative proteomics research and current applications in asthma. Expert review of proteomics, 18(12), 1045–1057. https://doi.org/10.1080/14789450.2021.2017777
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


