scRNA-seq Explained: Workflow, Tools, and Applications
Introduction: What is scRNA-seq and Why Did It Revolutionize Transcriptomics?
Single-cell RNA sequencing (scRNA-seq) is a cutting-edge technology that isolates individual cells from tissues or blood, constructs sequencing libraries for each cell, and quantifies gene expression at single-cell resolution. Modern high-throughput scRNA-seq platforms can profile thousands to tens of thousands of cells simultaneously, enabling unprecedented insights into cellular heterogeneity. Unlike bulk RNA-seq, which averages gene expression across cell populations, scRNA-seq uncovers rare cell types, dynamic cellular states, and stochastic gene expression patterns. This breakthrough has transformed fields such as developmental biology, immunology, neuroscience, and cancer research.
The revolutionary power of scRNA-seq lies in its ability to overcome the limitations of traditional transcriptomics. For example, it has revealed novel cancer cell subtypes in tumor microenvironments, mapped intricate cell fate trajectories during development, and decoded dynamic immune responses in T and B cells. By bridging fundamental biology and precision medicine, scRNA-seq has redefined our understanding of complex biological systems.
How scRNA-seq Works: From Single-Cell Isolation to Sequencing
The core workflow of scRNA-seq involves:
1. Single-cell isolation: Cells are separated via fluorescence-activated cell sorting (FACS), microfluidics (e.g., 10x Genomics), or laser microdissection and so on.
|
Technology/Method |
Advantages |
Disadvantages/Limitations |
|
Limiting Dilution |
- |
Only ~1/3 of wells obtain single cells at 0.5 cells/dilution; Low efficiency |
|
Micromanipulation |
- |
Time-consuming and low-throughput |
|
Flow Cytometry (FACS) |
Enables cell selection by markers |
Requires large input volume (difficult with <10,000 cells); Requires monoclonal antibodies |
|
Laser Capture Microdissection |
Enables targeted cell selection (2D) |
Low throughput |
|
Microfluidics |
Low sample consumption, cost-effective, precise fluid control |
Requires >1,000 cells; Restricted by homogeneous cell size requirements |
|
Microdroplet-based Microfluidics |
Small system volume; Enables low-cost manipulation of thousands to millions of cells |
- |
|
Microwell Technology |
Cost-effective and high-throughput |
Limited by Poisson distribution statistics |
2. Cell lysis and RNA capture: mRNA from each cell is captured using barcoded beads in droplet-based systems (e.g., Drop-seq), ensuring traceability to individual cells.
3. Reverse transcription and library preparation: mRNA is converted to cDNA, tagged with cell-specific barcodes and sequencing adapters, and amplified for sequencing.
4. High-throughput sequencing: Libraries are sequenced (e.g., Illumina) to generate gene expression profiles per cell.
5. Data analysis: Bioinformatics tools (e.g., UMAP, clustering algorithms) dissect cellular heterogeneity, identify subpopulations, and reconstruct developmental trajectories.
scRNA-seq Key Platforms and Technologies
The types of single-cell transcriptome sequencing technologies can be primarily categorized based on the range of transcript sequences captured during sequencing.
1. Full-length transcript sequencing technologies (e.g., Smart-seq2, MATQ-seq, SUPeR-seq, etc.):
Advantages:
- Capable of sequencing full-length transcripts.
- More sensitive and accurate in detecting gene expression.
- Suitable for various types of transcriptome sequencing data analysis.
Disadvantages:
- Low cell throughput.
- Relatively expensive.
2. 3′ or 5′-end sequencing technologies(e.g., Drop-seq, Seq-Well, Chromium, DroNC-seq, STRT-seq, etc.):
Advantages:
- High cell throughput.
- Cost-effective.
Disadvantages:
- Only sequences one end of the transcript.
- Lower sensitivity in detecting gene expression.
- Unsuitable for analyses such as alternative splicing or allele-specific expression.
scRNA-seq Data Analysis Steps
1. Raw data processing:
Demultiplexing by cell barcodes and UMIs.
Alignment (STAR, Cell Ranger) to reference genomes.
2. Quality control (QC):
Filtering low-quality cells (high mitochondrial genes, low UMI counts).
Generating a cell × gene count matrix.
3. Normalization & batch correction:
SCTransform or LogNormalize to adjust sequencing depth.
Harmony/Seurat CCA to mitigate batch effects.
4. Dimensionality reduction & clustering:
Visualization via PCA/t-SNE/UMAP.
Clustering (Louvain algorithm) to identify subpopulations.
5. Cell type annotation:
Marker gene analysis (CellMarker, PanglaoDB).
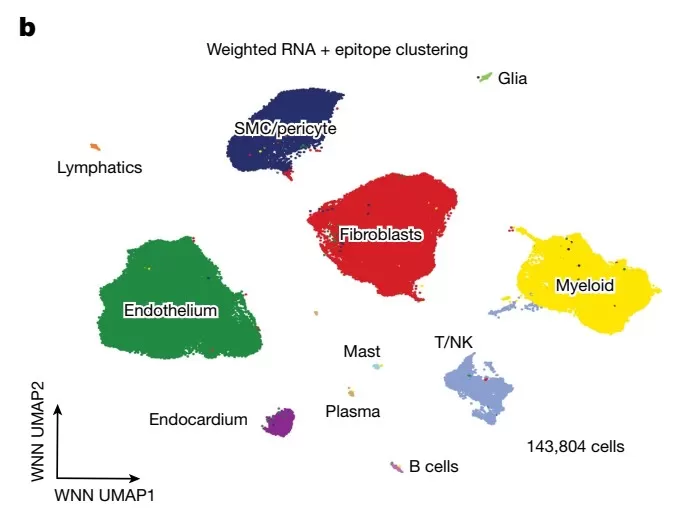
Figure 1: Heart tissue: 11 distinct cell types
6. scRNA-seq Advanced analyses:
Pseudotime trajectory inference (Monocle, Slingshot).
![Figure2:Common seven types of cell trajectory paths[2] Figure2:Common seven types of cell trajectory paths[2]](https://www.metwarebio.com/uploads/202508/Figure2 Common seven types of cell trajectory paths_1756103226_WNo_1568d204.webp)
Figure 2: Common seven types of cell trajectory paths
![Figure3:Chord diagram visualizing the information flow strength of NOTCH signaling pathway from ECs to mesenchymal cells[3] Figure3:Chord diagram visualizing the information flow strength of NOTCH signaling pathway from ECs to mesenchymal cells[3]](https://www.metwarebio.com/uploads/202508/Figure3 Chord diagram visualizing the information flow strength of NOTCH signaling pathway from ECs to mesenchymal cells_1756103264_WNo_928d599.webp)
Figure 3: Chord diagram visualizing the information flow strength of NOTCH signaling pathway from ECs to mesenchymal cells
Challenges in scRNA-seq: Technical Limits, Batch Effects, and Cost–Scale Trade-offs
Despite its power, scRNA-seq faces hurdles:
Technical limitations: Low RNA capture efficiency (dropout events), amplification biases, and sparse data.
Analytical complexity: High-dimensional data, batch effects, and computational demands.
Biological ambiguity: Defining transitional cell states or rare populations (<1% abundance).
Cost-throughput trade-offs: Deep sequencing (sensitivity) vs. cell numbers (scale).
Multi-omics integration: Aligning scRNA-seq with epigenomic (scATAC-seq) or proteomic (CITE-seq) data remains challenging.
Emerging technologies (e.g., microfluidics, long-read sequencing) and algorithms aim to address these gaps.
scRNA-seq Applications in Development and Disease Research
1. Developmental Biology
(1) Cell fate decisions: Mapping embryonic lineage diversification (e.g., human/mouse gastrulation atlases).
Example: The November 2024 Nature Methodsarticle "A comprehensive human embryo reference tool using single-cell RNA-sequencing data" employed single-cell transcriptomics to address the following key questions:
Ⅰ. Integrated six published datasets (covering zygotes, pre-implantation embryos, in vitro cultured blastocysts, and CS7 gastrulas) to construct a unified UMAP atlas comprising 3,304 cells, using fastMNN for batch effect correction.
Ⅱ. Defined lineage trajectories: For the first time, demonstrated the continuous developmental progression from inner cell mass (ICM) to epiblast/hypoblast, and from trophectoderm (TE) to chorionic trophoblast cells (CTB/STB/EVT).
Ⅲ. Identified key transcriptional regulators (including DUXA, VENTX, and ISL1) through SCENIC analysis, validating lineage-specific regulatory networks.
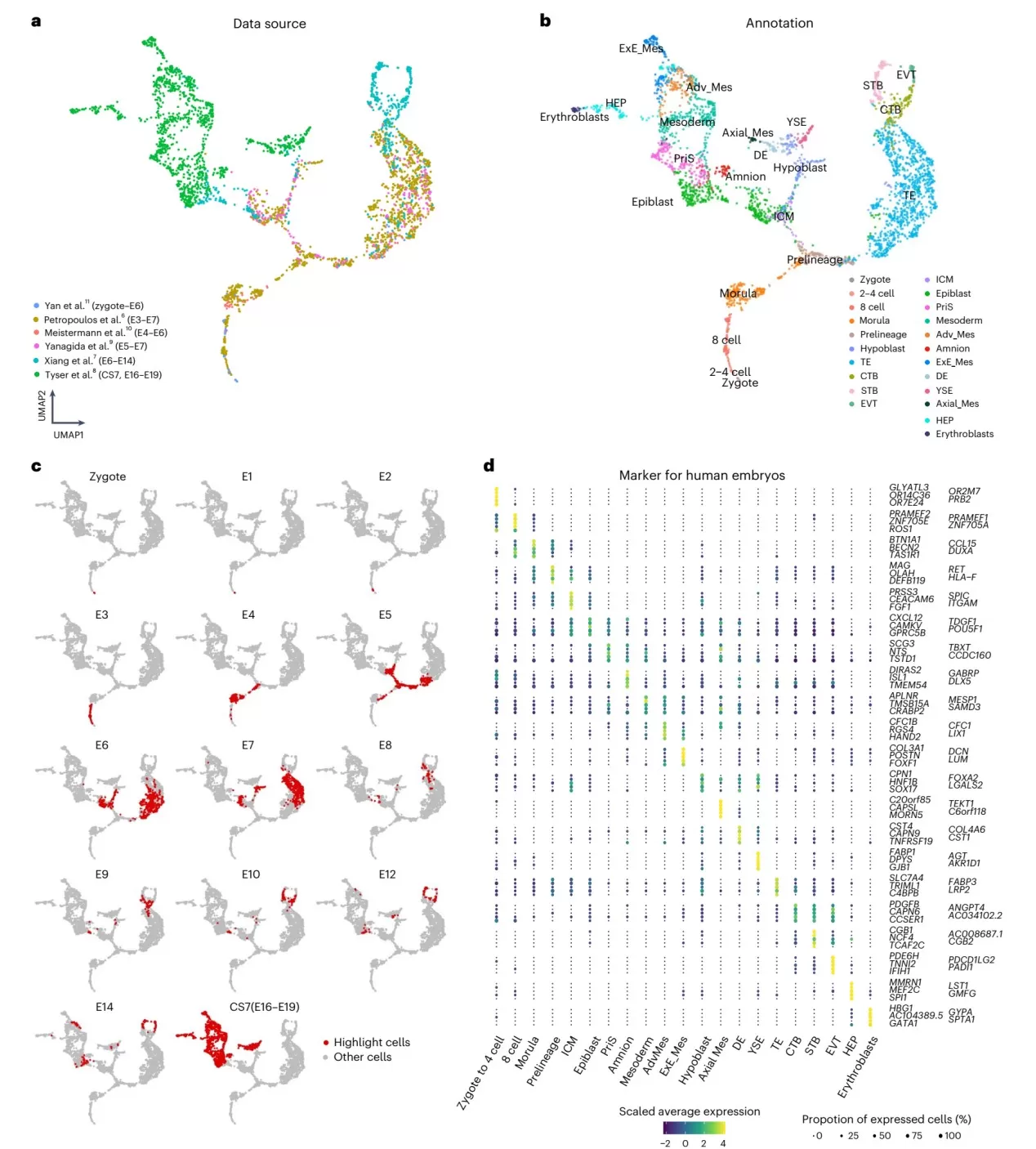
Figure 4: Human Embryo scRNA-seq Atlas: Integrated UMAP, Annotations, and Stage Markers
(2) Organogenesis: Decoding cellular heterogeneity in heart, brain, or liver development.
Example: The 2025 Nature Communications article "Heparan sulfate regulates myofibroblast heterogeneity and function to mediate niche homeostasis during alveolar morphogenesis" employed single-cell transcriptomics to address the following key questions:
Ⅰ. Through scRNA-seq analysis of postnatal day 5 (P5) mouse lung tissue, we identified two principal fibroblast populations: Wnt2⁺ alveolar fibroblasts (Alv-Fb) and Tgfbi⁺ myofibroblasts (MyoFb), with further subdivision revealing alveolar myofibroblasts (Alv-MyoFb-1/2) and ductal myofibroblasts (Ductal-MyoFb).
Ⅱ. Comparative scRNA-seq of control versus Ext1-knockout P8 lung tissue demonstrated:
- Significant reduction in the Alv-MyoFb-2 subpopulation
- Unaltered quantities of other fibroblast populations (e.g., Alv-Fb, Ductal-MyoFb)
- Decreased AT2 cell proportion alongside increased AT1 cell proportion in epithelial compartments
These findings suggest heparan sulfate (HS) non-autonomously regulates alveolar epithelial cell fate through fibroblast-mediated mechanisms.
_1756103650_WNo_1268d730.webp)
Figure 5: Heparan Sulfate/Ext1 Shapes Lung Myofibroblast Heterogeneity During Alveolar Morphogenesis (scRNA-seq)
(3) Stem cell differentiation: Tracing iPSC-derived neuron or cardiomyocyte trajectories.
2. Disease Mechanisms
(1) Cancer: Dissecting tumor microenvironments (malignant subclones, immune evasion).
Example: On January 2, 2025,Cancer Cell published online a research article entitled "Infiltrating Plasma Cells Maintain Glioblastoma Stem Cells through IgG-Tumor Binding", which employed single-cell transcriptomics to address the following key questions:
Ⅰ. Through scRNA-seq analysis of 6 GBM patient samples, the developmental trajectory of tumor-infiltrating B-cell lineages was identified, revealing abnormal enrichment of plasma cells (PCs, CD138⁺PRDM1⁺) in GBM.
Ⅱ. scBCR-seq demonstrated that PCs in GBM predominantly secrete IgG subtype antibodies (>50% proportion), with somatic hypermutation (SHM) rates significantly lower than those observed in other cancers.
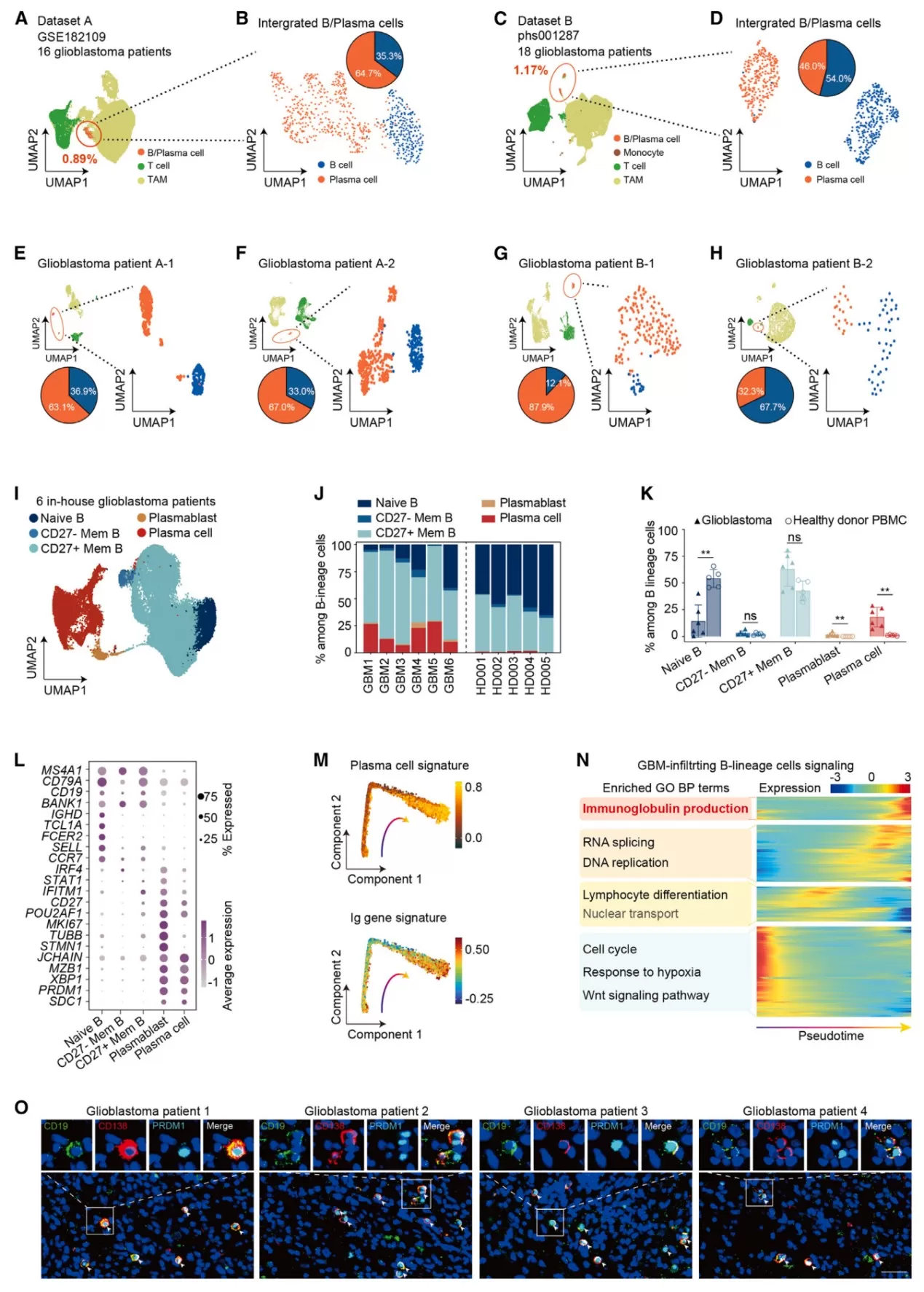
Figure 6: Glioblastoma B-Lineage Landscape Reveals Plasma-Cell Enrichment by scRNA-seq
(2) Neurodegeneration: Identifying dysregulated neuron/glia subsets in Alzheimer’s/Parkinson’s.
(3) Autoimmunity: Profiling immune dysfunction in lupus or rheumatoid arthritis.
3. Precision Medicine
(1) Rare diseases: Linking mutations to affected cell types.
(2) Drug discovery: Targeting tumor-specific neoantigens or immune checkpoints.
scRNA-seq represents a groundbreaking advancement in transcriptomics, enabling researchers to profile gene expression at unprecedented single-cell resolution.Despite its transformative potential, scRNA-seq faces several challenges including technical limitations in RNA capture efficiency, analytical complexities from high-dimensional data, and difficulties in rare cell population identification. Nevertheless, its applications span diverse research domains - from developmental biology and disease mechanisms to precision medicine - making it an indispensable tool in contemporary biomedical research. Ongoing innovations in microfluidics, sequencing technologies, and computational methods continue to expand its capabilities and clinical translation potential.
References
1. Amrute JM, Luo X, Penna V, et al. Targeting immune-fibroblast cell communication in heart failure. Nature. 2024;635(8038):423-433. doi:10.1038/s41586-024-08008-5
2. Saelens W, Cannoodt R, Todorov H, Saeys Y. A comparison of single-cell trajectory inference methods. Nat Biotechnol. 2019;37(5):547-554. doi:10.1038/s41587-019-0071-9
3. Xiang H, Pan Y, Sze MA, et al. Single-Cell Analysis Identifies NOTCH3-Mediated Interactions between Stromal Cells That Promote Microenvironment Remodeling and Invasion in Lung Adenocarcinoma. Cancer Res. 2024;84(9):1410-1425. doi:10.1158/0008-5472.CAN-23-1183
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


