Spatial Meets Single-Cell: The Future of Precision Omics
Introduction: Toward a Multidimensional Understanding of Biology
A multidimensional analysis of cellular diversity and tissue organization is essential to unravel how a multicellular organism develops from a single fertilized oosperm into a complex system composed of diverse cell types and functionally specialized tissues (Fig. 1A). It also sheds light on the regulatory mechanisms linking genomic information to specific biological processes. This comprehensive understanding can be organized around five key dimensions: (1) diversity of cell types; (2) abundance, proportion, and density of each cell type; (3) spatial organization and ecological interactions among cells; (4) temporal dynamics of cell state transitions; and (5) potential differentiation trajectories of distinct cell populations (Fig. 1B)[1].
The advent of high-throughput sequencing has revolutionized transcriptomic studies. Bulk RNA sequencing, which relies on homogenized tissue samples, has been widely used but obscures cell-specific expression profiles by averaging signals across heterogeneous cell populations. Since the first introduction of single-cell RNA sequencing (scRNA-seq) by Professor Tang Fuchou’s team in 2009, this technology has rapidly gained adoption for its ability to resolve cellular heterogeneity, leading to the identification of novel cell types and subtypes—particularly in cancer research, where it has revealed new immune and metabolically diverse tumor subpopulations.
However, while scRNA-seq excels at characterizing cellular composition, it loses critical spatial context. Techniques like immunohistochemistry (IHC) and in situ hybridization (ISH) offer spatial localization of specific targets but are limited to detecting only 10–100 molecules simultaneously. Spatial transcriptomics has emerged to overcome these limitations, enabling genome-wide expression profiling while preserving the native spatial architecture of tissues (Fig. 1C).
Integrating single-cell and spatial transcriptomics allows systematic investigation of biological processes—such as development and disease progression—across these five dimensions, providing a more holistic view of life’s functional mechanisms.
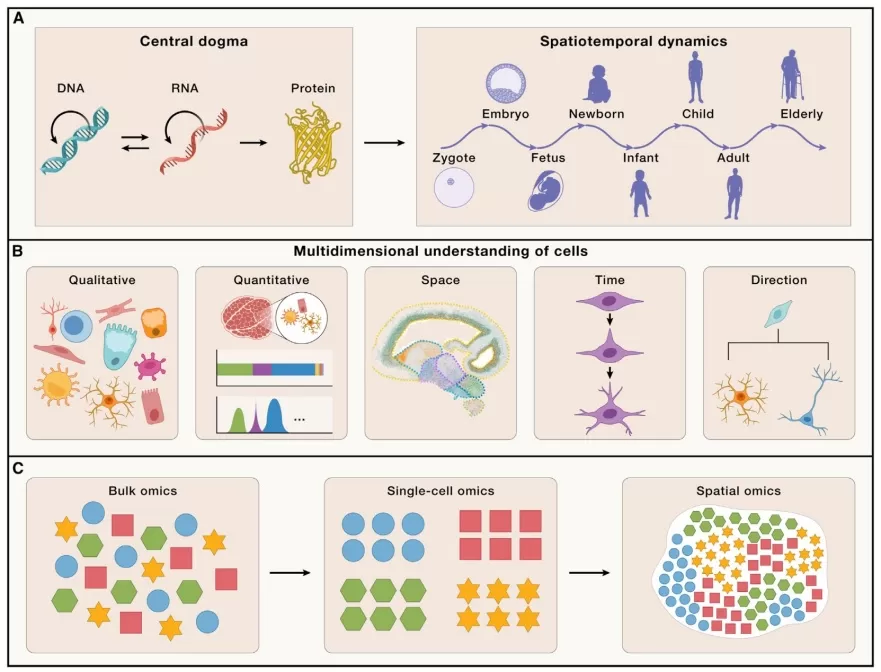
Figure 1. Multi-dimensional measurements of cells[1]
Why Integrate Spatial and Single-Cell Approaches?
Modern scRNA-seq platforms achieve remarkable cellular throughput; for example, 10x Genomics’ latest kits can capture nearly 20,000 cells per sample in a single run, greatly enhancing the resolution of cellular heterogeneity. A significant limitation, however, is that tissue dissociation during sample preparation destroys native spatial information. Since cellular function in multicellular organisms is deeply influenced by positional context within tissues, key questions—such as regional tissue characteristics, cellular community composition, and spatial interactions—require accurate spatial mapping.
Although current spatial transcriptomic technologies (e.g., Stereo-seq, 10x Visium HD) now offer single-cell resolution, they still underperform scRNA-seq in transcript capture sensitivity and coverage due to constrained capture areas and suboptimal biochemical efficiency. As a result, cell type identification or regional annotation based solely on spatial data remains challenging. Recent studies demonstrate that integrating scRNA-seq data significantly improves the interpretation and biological utility of spatial datasets.
Synergies and Use Cases: Aging, Tumor Metabolism, and Fibroblast Atlases
(1) Aging Mechanisms and Therapeutic Targets
A study published in Cell(November 4, 2024), titled “Spatial Transcriptomic Landscape Unveils Immunoglobin-Associated Senescence as a Hallmark of Agin”[2], combined spatial metabolomics and immunofluorescence to analyze multiple tissues from male mice across ages. It revealed that IgG accumulation is a key driver of aging and suggested that reducing IgG expression may ameliorate systemic aging.
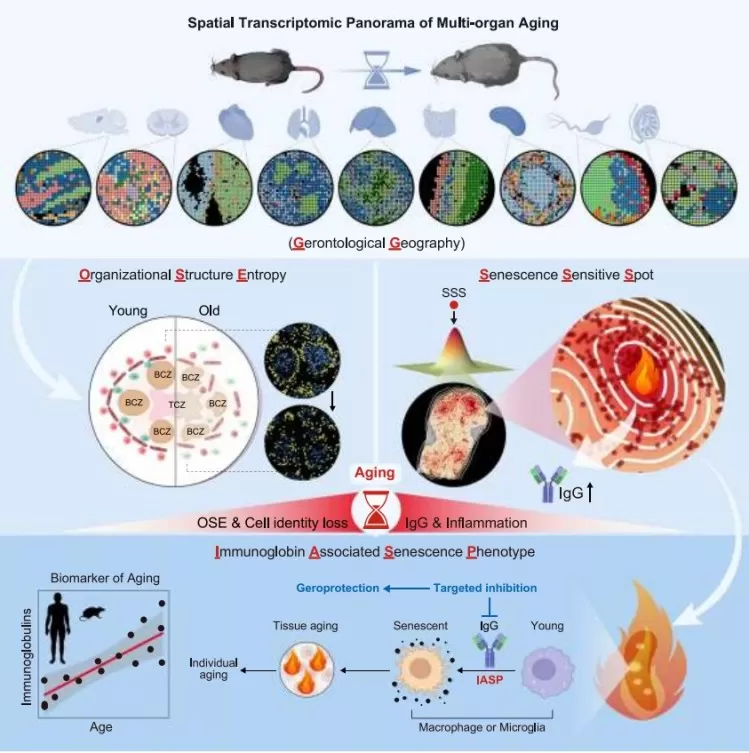
Figure 2. Spatial Transcriptomics Map of Multi-organ Aging
(2) Glucose Metabolism in Tumor Progression and Metastasis
Another Cellpaper (July 15, 2025),”Glucose Restriction Shapes Pre-Metastatic Innate Immune Landscapes in Lung through Exosomal TRAIL”[3], employed scRNA-seq, spatial transcriptomics, bulk RNA-seq, and multiplex immunofluorescence to investigate how glucose deprivation influences liver cancer metastasis to the lungs. The work uncovered a dual role of glucose restriction: while it suppresses primary tumor growth, it promotes metastasis through mechanisms independent of proliferation.
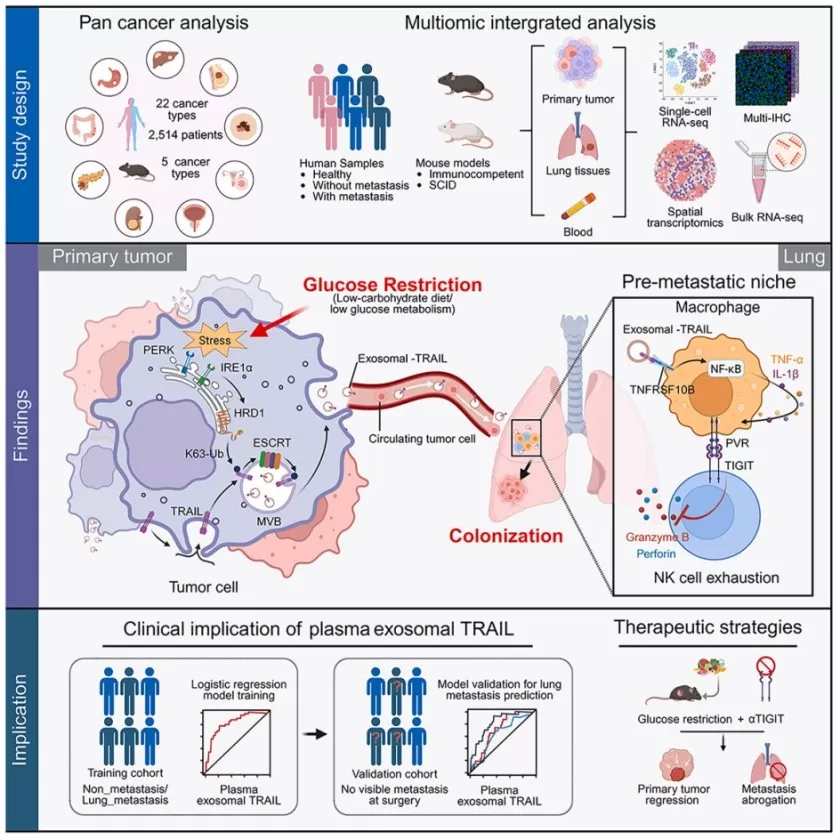
Figure 3. Multi-omics Study Glucose Restriction, Exosomal TRAIL, and Lung Metastasis
(3) Fibroblast Heterogeneity and Plasticity
A study in Cancer Cell(September 19, 2024), “Cross-Tissue Human Fibroblast Atlas Reveals Myofibroblast Subtypes with Distinct Roles in Immune Modulation”[4], leveraged scRNA-seq, spatial transcriptomics, and multiplexed immunoassays to profile fibroblast heterogeneity across 517 samples from 11 tissues. The findings offer new insights for developing targeted cancer therapies.
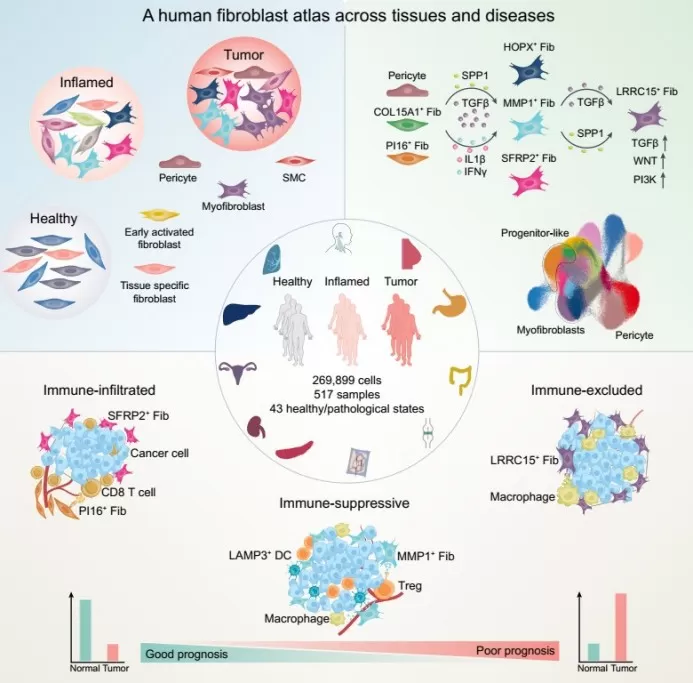
Figure 4. Human Fibroblast Atlas Across Tissues and Disease States
Key Tools for Spatial–Single-Cell Integration
The integration of scRNA-seq and spatial transcriptomics has become a pivotal strategy for deciphering cellular composition, spatial distribution, and interactions within tissue microenvironments. To combine high-resolution gene expression data with spatial context, numerous computational tools have been developed:
Deconvolution and mapping tools, such as Cell2location and SPOTlight use statistical or machine learning models to infer cell type proportions within each spatial spot;
Cross-modal alignment tools, like Tangram and Seurat employ transfer learning or deep learning to map single-cell data onto spatial expressions;
Integrated analysis framework, including Giotto and Squidpy support end-to-end workflows from cell type annotation to spatial gene imputation and interaction analysis.
Additional tools like RCTD and SpatialDWLS are optimized for cell typing in lower-resolution spatial data (e.g., standard Visium), while deep learning-based methods such as MUSE and DestVI excel at reconstructing fine-grained spatial cell state distributions.
Tool selection should be guided by the biological question and data properties. Researchers focused on spatial localization of cell types may prefer Cell2location or SPOTlight; those interested in imputing gene expression might choose Tangram or MUSE; and integrated analyses are best supported by Giotto or Squidpy. As demand for multimodal integration grows, these tools continue to evolve, deepening our understanding of spatial biology.
Challenges and Future Opportunities
1. Key Technical and Scientific Challenges
Balancing sensitivity, resolution, and throughput: Current spatial technologies still suffer from low gene capture efficiency and limited transcript detection at single-cell resolution. Multimodal integration is further complicated by technical noise and data sparsity, especially in spatial epigenomics and proteomics.
Computational and algorithmic limitations: Lack of standardized frameworks for cross-modal integration reduces reproducibility and comparability across tools. Processing large multimodal datasets requires substantial computational resources, and algorithms for 3D reconstruction and dynamic modeling remain underdeveloped.
Sample preparation and experimental design: Tissue dissociation, freezing, and sectioning can introduce RNA degradation or spatial distortion. Complex experimental designs involving multiple time points or samples are costly and difficult to apply to precious clinical specimens (e.g., FFPE tissues).
Biological interpretation and functional validation: Spatial co-localization does not prove functional interaction. Inferred cell communication networks must be validated experimentally—e.g., via spatial multi-omics or perturbational assays. Rare cell types and transitional states remain particularly challenging to capture and annotate.
2. Future Applications and Directions
Spatiotemporal cell atlas construction: Integrated technologies will enable dynamic, high-resolution mapping of cell types and states across time and space, advancing fundamental research in embryogenesis, organogenesis, and regenerative medicine.
Disease mechanisms and precision medicine: In cancer research, these approaches can elucidate spatial immune-tumor interactions, drug-resistant niches, and cellular ecosystem evolution. In neuroscience, they may help map region-specific neuron-glia networks and uncover spatial heterogeneity in degenerative or inflammatory diseases—ultimately contributing to biomarker discovery and targeted therapies.
Multimodal and multiomics integration: Future efforts will incorporate epigenomic, proteomic, and metabolomic data within a spatial context, enabling truly multi-layered functional dissection of cellular mechanisms in situ.
3D spatial reconstruction and in silico modeling: Combining high-resolution imaging with computational approaches (e.g., deep learning-based spatial reconstruction) will allow 3D transcriptomic mapping of tissues and organs, leading to more realistic models of cellular behavior and microenvironmental crosstalk.
Reference
[1] Liu L, Chen A, Li Y, Mulder J, Heyn H, Xu X. Spatiotemporal omics for biology and medicine. Cell. 2024 Aug 22;187(17):4488-4519.
[2] Ma S, Ji Z, Zhang B, Geng L, Cai Y, Nie C, Li J, Zuo Y, Sun Y, Xu G, Liu B, Ai J, Liu F, Zhao L, Zhang J, Zhang H, Sun S, Huang H, Zhang Y, Ye Y, Fan Y, Zheng F, Hu J, Zhang B, Li J, Feng X, Zhang F, Zhuang Y, Li T, Yu Y, Bao Z, Pan S, Rodriguez Esteban C, Liu Z, Deng H, Wen F, Song M, Wang S, Zhu G, Yang J, Jiang T, Song W, Izpisua Belmonte JC, Qu J, Zhang W, Gu Y, Liu GH. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell. 2024 Nov 27;187(24):7025-7044.e34.
[3] Wu CY, Huang CX, Lao XM, Zhou ZW, Jian JH, Li ZX, Wu YY, Liu ZY, Chen L, Liu L, Zheng L, Wei Y, Kuang DM. Glucose restriction shapes pre-metastatic innate immune landscapes in the lung through exosomal TRAIL. Cell. 2025 Jul 8:S0092-8674(25)00728-7.
[4] Gao Y, Li J, Cheng W, Diao T, Liu H, Bo Y, Liu C, Zhou W, Chen M, Zhang Y, Liu Z, Han W, Chen R, Peng J, Zhu L, Hou W, Zhang Z. Cross-tissue human fibroblast atlas reveals myofibroblast subtypes with distinct roles in immune modulation. Cancer Cell. 2024 Oct 14;42(10):1764-1783.e10.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


