Bile Acids and Human Disease: Metabolism, FXR/TGR5 Signaling, and Clinical Applications
Bile acids (BAs) are not only the body’s detergents for fat digestion; they are hormone-like signals that shape glucose and lipid metabolism, immunity, and the gut–liver axis. Synthesized from cholesterol in the liver and recycled through the enterohepatic circulation, bile acids activate FXR and TGR5 to maintain metabolic homeostasis. Dysregulated bile acid metabolism is linked to cholestasis, NAFLD/NASH, obesity, diabetes, and gut dysbiosis. This article explains what bile acids are, primary vs secondary bile acids, how they circulate and signal, their roles in liver and metabolic disease, how they interact with the microbiome, and how LC–MS bile acid profiling advances research and clinical translation.
Bile Acids Explained: Classification, Circulation, and Functions
What are bile acids (and bile salts vs bile acids)?
Bile acids are 24-carbon steroid acids derived from cholesterol in the liver and secreted into bile. Before secretion they are usually conjugated to glycine or taurine, forming water-soluble bile salts that remain ionized in the intestinal lumen. Strictly, “bile acids” are the protonated acids; “bile salts” are their deprotonated, conjugated forms. In practice, the terms are often used interchangeably in physiology and clinical labs.
Primary vs secondary bile acids
Primary bile acids—mainly cholic acid (CA) and chenodeoxycholic acid (CDCA)—are synthesized de novo by the liver. In the colon, microbiota deconjugate and 7α-dehydroxylate primary BAs to form secondary bile acids such as deoxycholic acid (DCA) from CA and lithocholic acid (LCA) from CDCA; ursodeoxycholic acid (UDCA) is a 7β-epimer of CDCA with therapeutic use. Secondary BAs tend to be more hydrophobic and display distinct receptor activities (e.g., potent TGR5 agonism).
Bile acids enterohepatic circulation (bile acid recycling)
After aiding digestion in the small intestine, ~95% of bile salts are actively reabsorbed in the terminal ileum (ASBT), returned to the liver via the portal vein, taken up by NTCP/OATPs, and resecreted into bile via BSEP, completing multiple cycles per day. For a step-by-step view of synthesis, transporters (NTCP, BSEP, ASBT), and feedback (FXR–FGF19), see the Pathways section below.
Bile acids functions: digestion and endocrine signaling
Digestion & cholesterol disposal: Amphipathic bile salts emulsify dietary fats into micelles, enabling absorption of fatty acids, cholesterol, and fat-soluble vitamins, and provide a route to eliminate excess cholesterol.
Antimicrobial activity: BAs suppress small-intestinal bacterial overgrowth and shape microbial communities.
Hormone-like signaling: BAs activate FXR (liver/ileum) and TGR5 (L-cells, adipose, macrophages). FXR restrains BA synthesis (↓CYP7A1/↓CYP8B1 via SHP and ileal FGF19), enhances export (↑BSEP), and improves hepatic glucose/lipid handling. TGR5 promotes GLP-1 secretion, energy expenditure, and anti-inflammatory effects.
Summary table: major human bile acids
|
Bile acid (salt) |
Type |
Key origin/metabolism |
Notable properties |
|
Cholic acid (CA) |
Primary |
Classic pathway; requires CYP8B1 (12α-OH) |
Strong detergent; precursor of DCA; higher 12α-OH BA fraction links to rapid proximal fat absorption |
|
Chenodeoxycholic acid (CDCA) |
Primary |
Classic & alternative pathways |
Potent FXR agonist; precursor of LCA/UDCA |
|
Deoxycholic acid (DCA) |
Secondary |
CA → 7α-dehydroxylation (microbial) |
FXR/TGR5 active; high levels can injure colonic epithelium |
|
Lithocholic acid (LCA) |
Secondary |
CDCA → 7α-dehydroxylation |
Very hydrophobic; immunomodulatory; potentially hepatotoxic when excessive |
|
Ursodeoxycholic acid (UDCA) |
Secondary (minor in humans) |
7β-epimer of CDCA |
Therapeutic BA for cholestasis; hydrophilic, low toxicity |
|
Glycine/Taurine conjugates (e.g., GCA, TCA) |
Conjugated salts |
Hepatic conjugation to glycine/taurine |
High solubility; efficient micelle formation; active ileal uptake |
Bile Acid Synthesis and Metabolism: Pathways at a Glance
From cholesterol to cholic acid: Hepatic bile acid synthesis begins with cholesterol. The classic pathway (ER/peroxisome; ~14 steps) is initiated by CYP7A1 (cholesterol 7α-hydroxylase), the rate-limiting step. Addition of a 12α-hydroxyl by CYP8B1 steers products toward cholic acid (CA); without CYP8B1, the pathway favors chenodeoxycholic acid (CDCA). In parallel, the alternative (acidic) pathway starts with mitochondrial CYP27A1 side-chain oxidation (notably in extrahepatic tissues) and contributes mainly to CDCA. After core transformations, bile acids are conjugated (glycine/taurine) and secreted into bile via BSEP.
Homeostatic control: Because bile acids can be cytotoxic when excessive, the liver–intestine axis uses FXR-FGF19/SHP feedback to restrain synthesis (↓CYP7A1/↓CYP8B1), enhance canalicular export (↑BSEP), and limit hepatocellular uptake—keeping the pool size safe yet sufficient for digestion.
Enterohepatic loop: After aiding fat absorption, conjugated bile salts are reabsorbed in the terminal ileum (via ASBT), returned to the liver through portal blood, taken up by NTCP/OATPs, and resecreted—repeating several times daily. Disruption of reabsorption increases colonic bile acids (bile acid diarrhea) and forces hepatic cholesterol consumption to replenish the pool.
Key components:
- CYP7A1: Classic pathway rate-limiting enzyme committing cholesterol to bile acid synthesis.
- CYP8B1: Sets the CA:CDCA ratio (12α-hydroxylation); a lever for metabolic effects.
- CYP27A1: Initiates the alternative pathway; defects cause BA synthesis disorders.
- FXR (± FGF19/SHP): Master regulator of bile acid homeostasis and hepatic lipid/glucose metabolism.
- TGR5 (GPBAR1): BA-sensing GPCR linking bile acids to GLP-1, energy expenditure, and anti-inflammation.
- Transporters: BSEP (canalicular export), NTCP/OATPs (hepatic uptake), ASBT (ileal uptake), OSTα/β (basolateral efflux).
Together, these enzymes, receptors, and transporters coordinate synthesis and recycling to balance digestion efficiency with cellular safety.
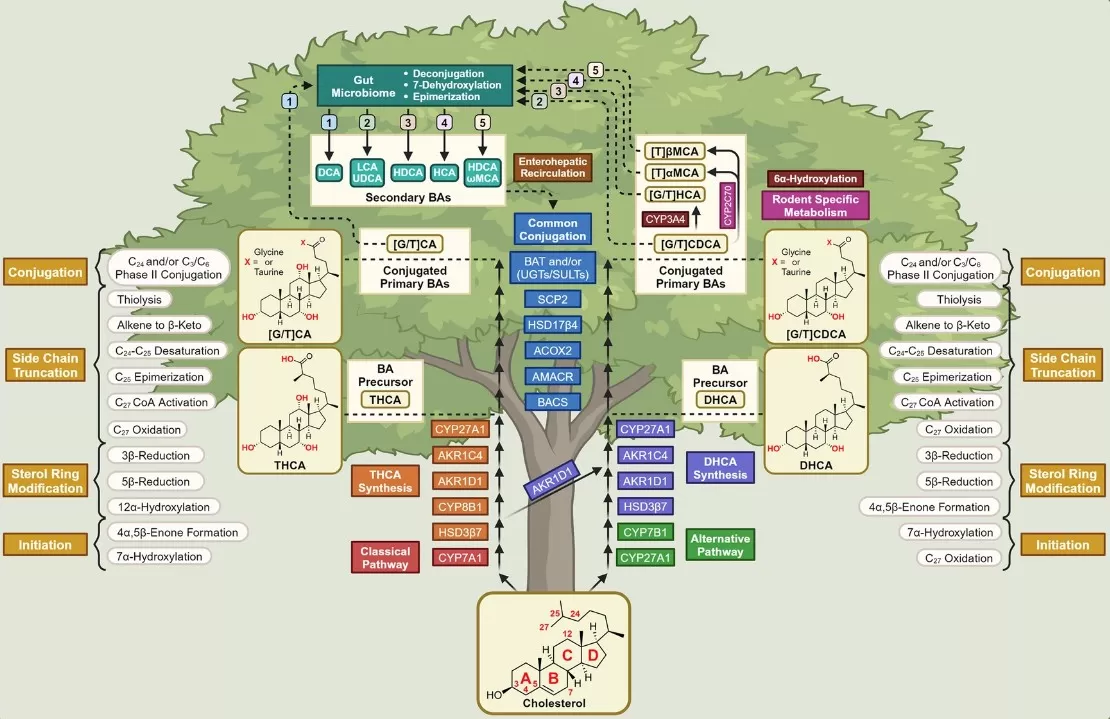
Biochemical Synthesis and Maturation of Bile Acids (Figure 1, Fleishman & Kumar, 2024)
Source: Fleishman JS, Kumar S. ‘Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets,’ Signal Transduction and Targeted Therapy (2024) 9:97. Figure 1. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Bile Acids in Liver Disease: From Cholestasis to Therapy
Bile acids have a double-edged role in the liver. In health, they aid nutrient absorption and, via FXR–FGF19/SHP feedback, restrain their own synthesis. In disease, impaired transport or bile flow (cholestasis) causes hepatocellular accumulation of toxic, hydrophobic bile acids, driving oxidative stress, inflammation, and fibrosis. This pattern is typical of primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and intrahepatic cholestasis of pregnancy (ICP), where patients often present with pruritus and elevated total bile acids in serum (a key diagnostic marker, especially in ICP).
Mechanistically, excessive bile acids activate inflammatory pathways and hepatic stellate cells, promoting scar formation. Conversely, FXR activation in hepatocytes and non-parenchymal cells reduces bile acid synthesis (↓CYP7A1/↓CYP8B1), enhances BSEP-mediated export, and dampens inflammation and fibrogenesis.
Therapeutically, bile acid pathways are actionable. Ursodeoxycholic acid (UDCA)—a hydrophilic, low-toxicity bile acid—is first-line in PBC and used in other cholestatic settings; it dilutes toxic bile acids and improves bile flow and biochemistry. Obeticholic acid (OCA), a potent FXR agonist, is approved as second-line for PBC and has shown anti-fibrotic signals in NASH, though pruritus and LDL increases require careful risk–benefit management. Beyond receptor agonism, compositional tuning of the bile acid pool is emerging: reducing 12α-hydroxylated bile acids (e.g., via CYP8B1 modulation) can improve glucose tolerance and steatosis in preclinical NAFLD/NASH by shifting intestinal nutrient handling and incretin responses.
In sum, bile acids are both perpetrators and targets in liver disease. Diagnostics (e.g., the total bile acids test) and therapies that modulate FXR signaling, transport, or bile acid composition offer complementary routes to protect the liver and slow disease progression.
Physiology of enterohepatic FXR–FGF19 signaling and its dysregulation in cirrhosis (Graphical abstract).
Source: Simbrunner B, Hofer BS, Schwabl P, et al. ‘FXR-FGF19 signaling in the gut–liver axis is dysregulated in patients with cirrhosis and correlates with impaired intestinal defence,’ Hepatology International (2024) 18:929–942. Graphical abstract. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Intrahepatic Cholestasis of Pregnancy (ICP): Diagnosis and UDCA Treatment
ICP is a pregnancy-specific cholestatic disorder, typically in late second/third trimester, presenting with nocturnal pruritus and elevated serum total bile acids. Diagnosis is supported by increased BA concentrations with a shift toward tauro- and glyco-conjugates; aminotransferases may be mildly to moderately raised. Species-resolved LC–MS/MS bile acid profiling refines risk assessment, distinguishes ICP from viral hepatitis, preeclampsia–HELLP, or drug-induced cholestasis, and provides a quantitative baseline for follow-up. Ursodeoxycholic acid (UDCA) is the first-line therapy: it dilutes hydrophobic BAs, improves canalicular flow, and alleviates pruritus while supporting transaminase normalization. Serial bile acid testing tracks biochemical response and informs obstetric teams alongside fetal surveillance policies. Most cases resolve within weeks postpartum, but recurrence in subsequent pregnancies is common; underlying variants in transport and phospholipid genes (e.g., ABCB11, ABCB4) may contribute. For clinical and translational studies, an LC–MS/MS ICP panel reporting conjugated species, primary:secondary ratios, and 12α-OH fractions enables standardized monitoring of disease activity and treatment efficacy.
Bile Acids in Metabolic Disorders: FXR/TGR5 and Energy Homeostasis
Bile acids link nutrient absorption to endocrine control of glucose, lipids, and energy. Via FXR (liver/ileum) and TGR5 (L-cells, adipose, macrophages), they modulate gluconeogenesis, insulin sensitivity, triglycerides, GLP-1 release, and thermogenesis. In obesity, type 2 diabetes, and NAFLD, the bile acid pool often shifts toward 12α-hydroxylated bile acids (e.g., cholic acid, DCA), which correlates with insulin resistance. Activating FXR generally suppresses CYP7A1/CYP8B1, lowers hepatic glucose production and triglyceride synthesis, and improves insulin signaling, though systemic FXR agonism can raise LDL—motivating tissue-selective strategies. TGR5 complements FXR by stimulating GLP-1 and increasing energy expenditure; excessive or mislocalized TGR5 signaling may have trade-offs in advanced NASH.
Therapeutically, bile acid pathways are actionable: FXR agonists (e.g., obeticholic acid) target NASH fibrosis; bile acid sequestrants (colesevelam) lower LDL and modestly improve glycemia; CYP8B1 inhibition shifts the pool toward CDCA-dominant species, enhancing distal fat delivery and incretin responses in preclinical models; TGR5 agonists remain investigational. In short, adjusting bile acid composition and signaling offers multi-lever control of metabolic disease—affecting how we absorb calories, signal nutrient status, and expend energy.
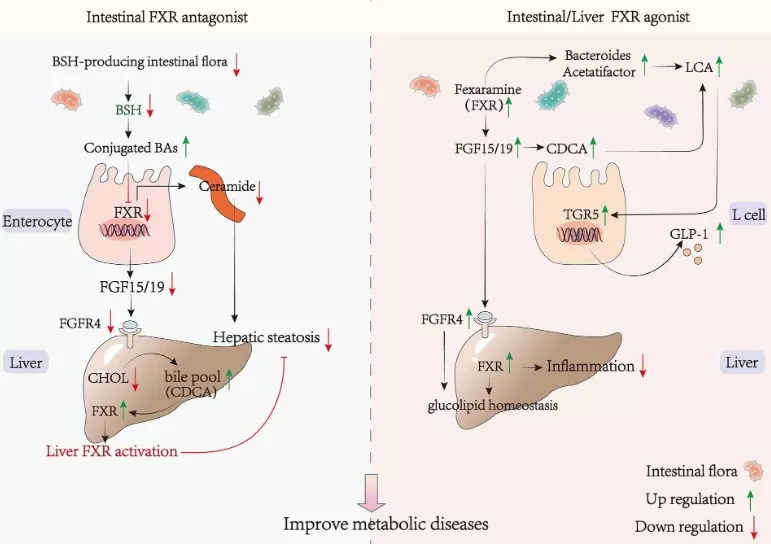
FXR–FGF15/19 Axis and Metabolic Disease Modulation (Figure 3, Frontiers in Nutrition, 2024).
Source: Li Y, Wang L, Yi Q, Luo L, Xiong Y. ‘Regulation of Bile Acids and Their Receptor FXR in Metabolic Diseases,’ Frontiers in Nutrition (2024) 11: 1447878. Figure 3. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Bile Acids and the Gut Microbiome: A Two-Way Street
The gut microbiome reshapes the bile acid pool, and bile acids, in turn, shape microbial ecology. Primary bile acids (CA, CDCA) enter the intestine mostly conjugated; microbes deconjugate and 7α-dehydroxylate them into secondary bile acids (e.g., DCA from CA, LCA from CDCA). These secondary species differ in receptor potency—some strongly engage FXR/TGR5, influencing metabolism, mucosal immunity, and inflammation. LCA derivatives can promote Treg differentiation, while excess DCA damages colonic epithelium and is linked to tumorigenic risk. Diet, antibiotics, and fiber rapidly shift both microbiota and bile acid profiles; fiber increases fecal BA loss and can lower LDL.
Bile acids also act as selective pressures: high luminal BA favors bile-tolerant taxa (e.g., Clostridium scindens), whereas reduced flow or BA binding alters community structure. Clinically, secondary BA depletion is common in IBD dysbiosis; conversely, raising colonic BAs with IBAT (ASBT) inhibitors improves constipation and can mimic some metabolic benefits of bariatric surgery via lower ileal FXR signaling. Emerging strategies—FMT, probiotics/enzymes, BA mimetics—seek to rebalance this axis. Net effect: tuning microbial BA transformation and host BA signaling offers a tractable path to modulate the gut–liver–metabolic network.
Because microbial BA conversions reshape FXR/TGR5 signaling, species-resolved LC–MS/MS bile acid panels provide quantitative readouts of primary/secondary shifts for IBD and NAFLD—see our targeted BA profiling for details.
_1761027527_WNo_488d425.webp)
Microbial Transformations of Human Bile Acids (Figure 2, Microbiome, 2021).
Source: Guzior DV, Quinn RA. ‘Review: Microbial Transformations of Human Bile Acids,’ Microbiome (2021) 9: 140. Figure 2. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Bile Acids in Daily Life: Digestion, LDL, and IBS-D
Bile acids (BAs) are central to everyday digestion: after a high-fat meal, hepatic secretion and gallbladder release of bile salts emulsify lipids, enabling absorption of fatty acids, cholesterol, and fat-soluble vitamins. After cholecystectomy, the continuous trickle of bile may reduce tolerance of large fatty meals, and a subset develop BA-related diarrhea. Soluble fiber (e.g., oats, fruits) and bile acid sequestrants (cholestyramine, colesevelam) bind luminal BAs, increase fecal BA loss, and can lower LDL cholesterol over time. In IBS-D and ileal disease/resection, bile acid diarrhea arises when excess BAs spill into the colon; sequestrants often relieve urgency and stool frequency. In the gallbladder, an unfavorable bile composition (low BA relative to cholesterol) promotes cholesterol gallstones; ursodeoxycholic acid (UDCA) can dissolve select radiolucent stones in carefully chosen patients. Beyond hepatogastroenterology, an injectable deoxycholic acid (DCA) formulation is used for small-area submental fat reduction, reflecting BA chemistry’s practical reach from digestion to lipid management and symptom control.
Bile Acid Testing & Our Targeted LC–MS Service
Why profile bile acids? Total bile acids tests are useful but lack specificity. Research and precision medicine need species-resolved, absolute quantification to distinguish primary vs secondary, conjugated vs unconjugated, and oxidized/sulfated derivatives.
LC–MS/MS bile acid profiling separates and quantifies dozens of BA species with high sensitivity/specificity using isotope-labeled internal standards and compound-specific calibration for absolute quantification.
MetwareBio Targeted Bile Acid Metabolomics (LC–MS):
- Broad coverage: 65+ bile acids, including CA, CDCA, DCA, LCA, UDCA and glyco/tauro conjugates, plus key oxo/sulfated species.
- Accuracy & reproducibility: Multiple internal standards; per-analyte calibration (typical R² > 0.99).
- Actionable outputs: Publication-ready concentration tables, QA/QC, and expert interpretation.
FAQ
Q1. What’s the difference between bile acids and bile salts?
Bile acids are the protonated acids; bile salts are their glycine/taurine-conjugated, deprotonated forms that dominate in bile/intestine. Conjugation raises solubility and efficacy for micelle formation and ileal transport.
Q2. How do gut bacteria transform bile acids?
Colonic anaerobes deconjugate and 7α-dehydroxylate primary BAs: CA→DCA, CDCA→LCA; others epimerize CDCA to UDCA. These secondary BAs have distinct FXR/TGR5 activities that affect host metabolism and immunity.
Q3. Do bile acids regulate glucose and lipids via FXR?
Yes. FXR activation reduces hepatic gluconeogenesis and triglyceride synthesis, improves insulin sensitivity, and restrains BA synthesis via FGF19/SHP. TGR5 complements this with GLP-1 release and thermogenesis.
Q4. Are bile acids useful biomarkers?
Clinically, total bile acids support cholestasis/ICP diagnosis. Research-grade LC–MS panels reveal disease-specific BA signatures (e.g., altered primary/secondary ratios in NAFLD/NASH, conjugate elevations with transporter dysfunction), improving stratification and mechanistic insight.
References
- Fleishman JS, Kumar S. Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9(1):97. doi:10.1038/s41392-024-01811-6.
- Collins SL, Stine JG, Bisanz JE, et al. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21(4):236-247. doi:10.1038/s41579-022-00805-x.
- Fuchs CD, Simbrunner B, Baumgartner M, Campbell C, Reiberger T, Trauner M. Bile acid metabolism and signalling in liver disease. J Hepatol. 2025;82(1):134-153. doi:10.1016/j.jhep.2024.09.032.
- Simbrunner B, Hofer BS, Schwabl P, et al. FXR-FGF19 signaling in the gut–liver axis is dysregulated in patients with cirrhosis and correlates with impaired intestinal defence. Hepatology International. 2024;18:929-942. doi:10.1007/s12072-023-10636-4.
- Li Y, Wang L, Yi Q, Luo L, Xiong Y. Regulation of bile acids and their receptor FXR in metabolic diseases. Front Nutr. 2024;11:1447878. doi:10.3389/fnut.2024.1447878.
- Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9:140. doi:10.1186/s40168-021-01101-1.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


_1761027259_WNo_685d331.webp)
