Glycophospholipids: The "Sugar-Fat" Lipids with Big Health Impacts
Introduction to Glycophospholipids in Human Health
Glycophospholipids are a little-known group of cellular metabolites that pack a big punch in biology and health. These molecules combine features of both fats and sugars – essentially “sugar-fat” lipids – and reside in cell membranes in tiny amounts. Despite being rare, they have attracted growing attention for their unique roles in cell signaling, immune regulation, and disease. In this blog, we will explore what glycophospholipids are, how they were discovered, how our bodies make and break them down, and why they matter for human health (with a nod to plants and bacteria too). By understanding these uncommon lipids, researchers and clinicians can gain new insights into brain development, immune cell function, and even potential therapies for diseases. Let’s dive into the sweet and fatty world of glycophospholipids!
Overview:
- What Are Glycophospholipids?
- Glycophospholipids Discovery and Structure: From Bacterial Oddty to Brain Lipid
- Glycophospholipids Biosynthesis: UGGT2-PA Glucosylation, GPI Anchors, and Bacterial Routes
- Glycophospholipids Metabolism in Animals: Turnover, Remodeling & Signaling
- Glycophospholipids in Human Health and Disease
- Glycophospholipids in Plant Cell Walls and Signaling
- Everyday Applications of Glycophospholipids: Diagnostics, Vaccines & Analytics
- How to Profile Glycophospholipids
- MetwareBio's Lipidomics: Enabling Glycophospholipid Discovery
What Are Glycophospholipids?
Glycophospholipids are hybrid membrane lipids built on a glycerol backbone bearing two fatty acyl chains and a phosphate group that is further glycosylated (a sugar is attached to the phosphate). In short, they are phospholipids with a carbohydrate headgroup. This sets them apart from classic glycerophospholipids (e.g., PC, PE, PI), whose headgroups are small alcohols without sugars, and from glyceroglycolipids (common in plants), which carry sugars without phosphate. Though typically low-abundance, glycophospholipids are functionally potent: they help organize membrane microdomains, modulate signaling, and serve as anchors (e.g., GPI anchors) for many cell-surface proteins. Representative molecules include phosphatidylglucoside (PtdGlc) in mammalian tissues and GPI lipids across eukaryotes. Terminology overlaps, so here’s a quick comparison: glycophospholipids carry both phosphate and sugar; glycerophospholipids have phosphate only; glycolipids have sugar only—clarity that improves LC–MS/MS lipidomics annotation.
|
Term |
Phosphate? |
Sugar? |
Typical examples |
Notes / Where common |
|
Glycophospholipids |
✔ |
✔ |
PtdGlc; GPI lipid |
Rare but functional; raft/microdomain roles |
|
Glycerophospholipids |
✔ |
✖ |
PC, PE, PS, PI |
Bulk membrane matrix; signaling precursors |
|
Glyceroglycolipids |
✖ |
✔ |
MGDG, DGDG |
Abundant in plant chloroplasts; no phosphate |
|
Glycosphingolipids |
✖ |
✔ |
GM1, GlcCer |
Neural membranes; classic “glycolipids” |
|
“Phosphoglycolipids” (usage varies) |
Often ✔ |
✔ |
E.g., bacterial ECA-PG |
A descriptive label; not a strict class name |

Headgroups and Backbones of Membrane Lipids: Where Glycophospholipids Fit (Structures of (A) phosphatidylcholine, (B) sphingomyelin, (C) monogalactosyldiacylglycerol, and (D) glucosylceramide)
Source: Osawa T, Fujikawa K, Shimamoto K. “Structures, Functions, and Syntheses of Glycero-Glycophospholipids,” Frontiers in Chemistry (2024) 12:1353688. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Glycophospholipids Discovery and Structure: From Bacterial Oddity to Brain Lipid
First evidence for glycophospholipids came in the 1970s with bacterial phosphatidyl-α-glucoside; three decades later, the β-anomer was detected in human umbilical-cord erythrocytes and purified from fetal mouse brain. Isolation leveraged antibody-guided membrane microdomain capture with selective phospholipases to remove confounding lipids, followed by NMR, GC, and high-resolution MS for structure elucidation. Hallmarks of phosphatidylglucoside (PtdGlc) include a diacylglycerol-1-phosphate-β-D-glucose headgroup, a minor O-acetylated species, uncommon glycerol stereochemistry (~15% S configuration), and a strikingly saturated acyl profile (18:0 at sn-1, 20:0 at sn-2). This deep saturation and stereochemistry favor tight packing and formation of dedicated membrane microdomains distinct from sphingolipid–cholesterol rafts. Biologically, PtdGlc shows developmental and cell-type specificity—enriched in neural progenitors/immature glia, scarce in adult neurons, and upregulated along the neutrophil lineage—supporting a role in organizing signaling platforms. Enzymatic hydrolysis can yield lyso-PtdGlc, a downstream signaling intermediate, without altering the defining structural features above.
Glycophospholipids Biosynthesis: UGGT2–PA Glucosylation, GPI Anchors, and Bacterial Routes
In mammals, glycophospholipids such as phosphatidylglucoside (PtdGlc) form when the ER enzyme UGGT2 transfers glucose from UDP-Glc to saturated phosphatidic acid (PA). This “lipid-quality-control” step is favored when desaturation (via SCD) is limited, and newly formed PtdGlc is cleared via lysosome–autophagy to alleviate ER lipid stress. In eukaryotes more broadly, GPI anchors are assembled on phosphatidylinositol through a multi-enzyme PIG/PGAP pathway (cytosolic initiation, ER-lumen mannose additions, ethanolamine-phosphate additions), then attached to proteins by GPI-transamidase and remodeled in the ER/Golgi. In Gram-negative bacteria, glycophospholipids arise via distinct routes: ECA uses an Und-P carrier and is finally transferred to phosphatidylglycerol (ECA_PG), whereas MPIase appears to build a pyrophosphate-linked glycan directly on DAG via CDP-DAG/CdsA chemistry. These complementary pathways can be profiled with LC-MS/MS lipidomics using diagnostic sugar-phosphate fragments for confident class annotation.
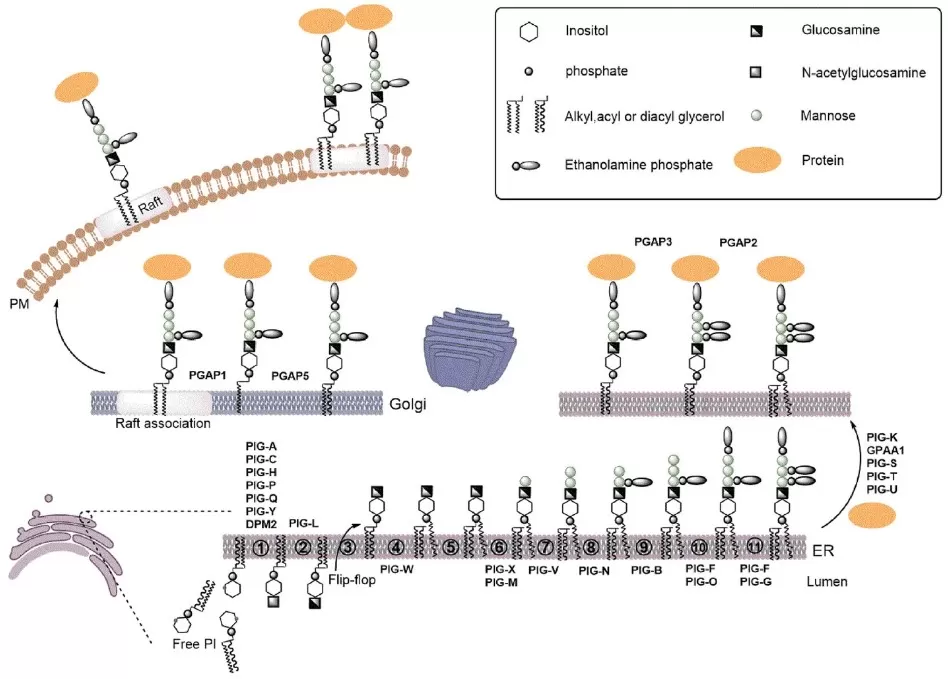
Overview of GPI-Anchor Biosynthesis, Attachment, and Remodeling
Source: Wu T, Yin F, Guang S, He F, Yang L, Peng J. “The Glycosylphosphatidylinositol biosynthesis pathway in human diseases,” Orphanet Journal of Rare Diseases (2020) 15:129, Figure 1. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
 and MPIase_1758856713_WNo_624d493.webp)
Biosynthetic Pathways of Enterobacterial Common Antigen (ECA) and MPIase
Source: Osawa T, Fujikawa K, Shimamoto K. “Structures, Functions, and Syntheses of Glycero-Glycophospholipids,” Frontiers in Chemistry (2024) 12:1353688, Figure 5. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Glycophospholipids Metabolism in Animals: Turnover, Remodeling & Signaling
Once synthesized, glycophospholipids are actively remodeled and cleared rather than being static membrane bricks. A key route is deacylation: phospholipase A activity converts phosphatidylglucoside (PtdGlc) to lyso-PtdGlc, which can be exported and act as a paracrine signal. In the developing nervous system, lyso-PtdGlc engages GPR55 on neurons to shape nociceptive neurite growth, illustrating how metabolism generates bioactive lipid messengers. Inside cells, lyso-species and intact PtdGlc are trafficked to endo-lysosomes where glycosidases and phospholipases hydrolyze the sugar, phosphate, and acyl chains to recyclable glycerol and fatty acids—flux that lipidomics can quantify with LC-MS/MS lipidomics. For glycosylphosphatidylinositol pathways, GPI-anchored proteins are shed by GPI-specific phospholipase D (GPI-PLD) or PI-specific PLC, released on vesicles/micelles, then cleared by hepatic endocytosis; the lipid/glycan fragments are subsequently degraded in lysosomes. Together, these routes tune membrane composition, generate signaling ligands, and prevent accumulation of potentially harmful saturated lipids—central readouts for modern lipidomics analysis.

Glycophospholipids metabolism in animals (Panel A:PtdGlc ↔ lyso-PtdGlc remodeling & signaling.Panel B:GPI-anchored proteins turnover).
Panel A Source: Osawa T, Fujikawa K, Shimamoto K. “Structures, functions, and syntheses of glycero-glycophospholipids,” Frontiers in Chemistry (2024) 12:1353688. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Panel B Source: Müller GA, Müller TD. “(Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins I,” Biomolecules (2023) 13(5):855. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Glycophospholipids in Human Health and Disease
Glycophospholipids may be tiny players in our cells, but research is revealing outsized impacts on health and disease. From genetic disorders that cripple GPI anchors, to the involvement of PtdGlc in immune cell death and neural growth, these metabolites touch on many aspects of human biology. Below, we highlight a few key discoveries from recent years that show how “sugar-phospholipids” influence human health.
Glycophospholipids in Inherited GPI Deficiency (IGD)
In IGD, glycophospholipids fail at a crucial job: building GPI-anchored proteins (GPI-APs) that organize synaptic signaling, complement control, and alkaline phosphatase activity. Pathogenic variants in PIG/PGAP genes disrupt assembly, attachment, or remodeling of the GPI glycophospholipid, yielding neurodevelopmental delay, seizures, hypotonia, and multisystem features. Clinically, flow cytometry (e.g., FLAER binding) profiles surface GPI-APs, while LC-MS/MS lipidomics analysis quantifies PI/GPI intermediates to pinpoint pathway blocks and support variant interpretation. Mechanistically, loss or mislocalization of raft-resident GPI-APs derails receptor clustering and cell–cell communication; hepatic clearance of shed GPI-APs and lysosomal catabolism become compensatory but insufficient. Early case reports suggest genotype-tailored interventions (e.g., epigenetic up-regulation in select defects) can partially restore GPI output, illustrating that glycophospholipids pathways are druggable. Related research links phosphatidylglucoside (PtdGlc) to neutrophil apoptosis and glia–neuron signaling, but those belong in extended reading to keep this section focused on IGD-driven human disease.
–driven apoptosis in AML TUNEL quantification and caspase-3 act_1758856899_WNo_279d311.webp)
Glycophospholipids (PtdGlc)–driven apoptosis in AML: TUNEL quantification and caspase-3 activation
Source: Adapted from Yokoyama N, Ekyalongo RC, Kage M, et al. Phosphatidylglucoside regulates apoptosis of human neutrophilic lineage cells. Frontiers in Immunology. 2025;16:1597423. Figure 3C–D. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Glycophospholipids in Neutrophil Biology and AML
Glycophospholipids shape innate immunity through phosphatidylglucoside (PtdGlc) on maturing neutrophils. Engaging PtdGlc triggers caspase-8/-3–dependent apoptosis, a built-in timer that promotes resolution of inflammation. In acute myeloid leukemia (AML) models, an anti-PtdGlc antibody (DIM21) accelerates apoptosis and synergizes with ATRA—positioning PtdGlc as both a surface biomarker and a potential therapeutic target. Mechanistically, PtdGlc enriches specialized membrane microdomains (lipid rafts), facilitating death-receptor–independent signaling. For measurement, LC-MS/MS lipidomics analysis quantifies PtdGlc/lyso-PtdGlc alongside classical glycerophospholipids, while flow cytometry tracks surface expression to stratify samples. Together, these readouts link glycophospholipids to neutrophil lifespan control and reveal a druggable axis in myeloid malignancy.
 in AML Annexin VPI apoptosis quantification with ATRA synergy_1758856977_WNo_490d242.webp)
Glycophospholipids (PtdGlc) in AML: Annexin V/PI apoptosis quantification with ATRA synergy
Source: Adapted from Yokoyama N, Ekyalongo RC, Kage M, et al. Frontiers in Immunology. 2025;16:1597423. Figure 5E. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Glycophospholipids in the Nervous System and Pain Signaling
During development, glycophospholipids—notably PtdGlc—are enriched in neural stem cells and immature glia, where they help assemble membrane microdomains that cue astroglial differentiation. Enzymatic remodeling yields lyso-PtdGlc, which activates GPR55 on neurons to guide nociceptive axon growth, connecting glycophospholipid metabolism to pain-circuit wiring. In adult neurons, PtdGlc is scarce, but downstream signaling can persist via glia-derived ligands. Disruption of glycophospholipid pathways—especially GPI-anchored proteins (GPI-APs)—impairs synaptic organization and neurodevelopment. Quantitatively, LC-MS/MS lipidomics and MALDI imaging mass spectrometry profile PtdGlc/lyso-PtdGlc across brain regions, while proteomics of GPI-APs links lipid microdomains to receptor clustering. These tools reveal how glycophospholipids tune neural differentiation and pain signaling.
Glycophospholipids in Plant Cell Walls and Signaling
Plants rely on glycophospholipids primarily as GPI anchors that tether hundreds of proteins to the outer leaflet of the plasma membrane. These GPI-anchored proteins include arabinogalactan proteins and cell-wall-modifying enzymes, positioning catalysts exactly where cellulose and matrix polysaccharides are built. GPI-APs also partner with receptor-like kinases, confining receptors to membrane nanodomains and sharpening extracellular signal transduction. Loss of GPI biosynthesis causes severe growth defects, underscoring pathway essentiality. From an analytics standpoint, LC-MS/MS lipidomics can monitor PI/GPI intermediates, while shotgun lipidomics and MALDI imaging map class distributions across tissues. For ag-biotech, these data connect glycophospholipids to traits such as stress tolerance, root development, and pathogen response—actionable targets for breeding or gene-editing programs.
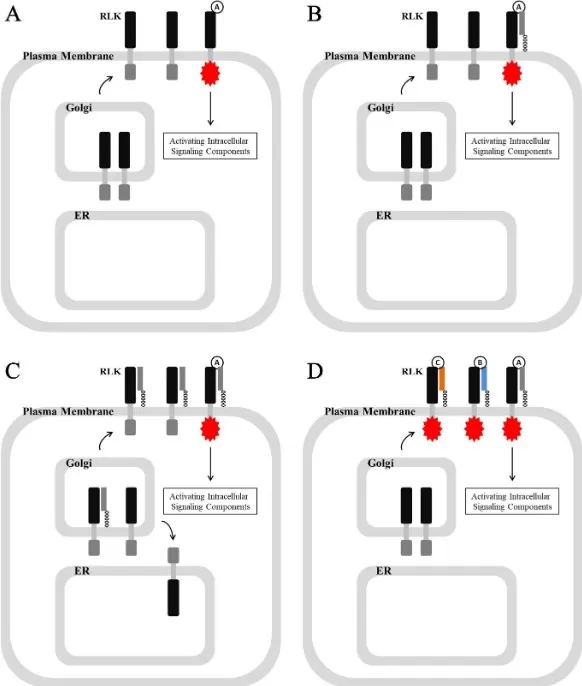
GPI-Anchored Protein–RLK Signaling at the Plant Cell Surface
Source: Zhou K. “Glycosylphosphatidylinositol-anchored proteins in Arabidopsis: common roles in signaling and development,” Frontiers in Plant Science (2019). Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Everyday Applications of Glycophospholipids: Diagnostics, Vaccines & Analytics
Though minor in abundance, glycophospholipids have clear applied value. GPI anchors and GPI-anchored proteins serve as cell-surface biomarkers and therapeutic targets, while phosphatidylglucoside (PtdGlc) is being explored for myeloid malignancy monitoring. In microbiology, enterobacterial glycophospholipids (e.g., ECA in the bacterial outer membrane) inform serodiagnostics and rational attenuated-vaccine design. Methodologically, lipidomics analysis—especially targeted LC-MS/MS lipidomics with MRM transitions to sugar-phosphate fragments—enables sensitive quantification across tissues and biofluids. MALDI imaging mass spectrometry localizes glycophospholipids in situ (e.g., within lipid rafts/membrane microdomains), while complementary shotgun lipidomics supports high-throughput screening. These workflows translate to real-world use cases: quality control in bioprocessing, disease stratification panels combining GPI intermediates with classical phospholipids, and mechanistic readouts for drug development that modulate raft signaling or glycophospholipid remodeling. In short, decoding glycophospholipids connects basic membrane chemistry to actionable diagnostics, vaccines, and precision-medicine analytics.
How to Profile Glycophospholipids
For rigorous glycophospholipids profiling, start with Folch or Bligh–Dyer extraction and add SPE enrichment to recover sugar-phosphate lipids. The core readout is LC-MS/MS lipidomics: HILIC/normal-phase LC separates glyco- vs non-glyco classes; diagnostic fragments (e.g., sugar-phosphate ions) enable confident ID, while MRM provides absolute quantification. For rapid surveys, shotgun lipidomics with precursor/neutral-loss scans flags Hexose-phosphate signatures—use fractionation to minimize ion suppression. Spatial context comes from MALDI imaging mass spectrometry (and SIMS) to map PtdGlc/GPI in tissue microdomains (lipid rafts). Use NMR of lipids or targeted hydrolysis for linkage/anomer confirmation when structures are novel. Data processing relies on LIPID MAPS/SwissLipids for annotation, with isotope-labeled standards, pooled-QC, and carryover checks to ensure reproducible lipidomics analysis. This practical toolkit captures low-abundance glycophospholipids with sensitivity, specificity, and spatial insight—and aligns directly with quantitative LC-MS/MS lipidomics and spatial metabolomics services.
MetwareBio’s Lipidomics: Enabling Glycophospholipid Discovery
Understanding and quantifying glycophospholipids can unlock insights into cell function and disease – and this is where professional metabolomics services like MetwareBio come in. MetwareBio’s comprehensive Quantitative Lipidomics platform is well-equipped to detect even trace lipids like glycophospholipids. Through state-of-the-art LC–MS/MS and rigorous bioinformatic analysis, we provide accurate identification and quantitation of these rare lipids across biological samples. Our team has optimized extraction and MS methods (including selective MRM modes for glycophospholipid markers) to ensure sensitivity and reliability. We also offer spatial metabolomics services – using imaging mass spectrometry to map lipid distributions in tissues – which can pinpoint where glycophospholipids localize in an organ. With extensive experience in lipidomics quality control and a constantly updated internal database, MetwareBio delivers high-confidence results. In short, whether you are investigating GPI-anchored protein deficiencies or novel glycolipid biomarkers, MetwareBio’s lipidomics services provide the cutting-edge tools and expertise to propel your research forward.
Glycophospholipids FAQs
Q1. What are glycophospholipids vs glycolipids vs glycerophospholipids?
Glycophospholipids carry both a phosphate and a sugar on a glycerol backbone; glycerophospholipids have phosphate only; glycolipids (e.g., glycosphingolipids, glyceroglycolipids) have sugar but no phosphate.
Q2. What is phosphatidylglucoside (PtdGlc) in glycophospholipids?
PtdGlc is a glycophospholipid enriched in membrane microdomains; it links to neutrophil apoptosis and neural development. Quantify it via LC-MS/MS lipidomics (diagnostic sugar-phosphate fragments).
Q3. How are GPI-anchored proteins made (glycophospholipids, GPI biosynthesis)?
In the ER, PIG/PGAP enzymes add sugars to PI and attach the GPI glycophospholipid to proteins, then remodel acyl chains for raft localization.
Q4. What is ECA in bacteria (glycophospholipids context)?
Enterobacterial Common Antigen (ECA) occurs as ECA_PG (on phosphatidylglycerol), ECA_LPS (on LPS), and ECA_CYC (cyclic, periplasmic); it strengthens the bacterial outer membrane.
Q5. Which methods best profile glycophospholipids?
Use LC-MS/MS lipidomics (HILIC/normal-phase) for ID/quant; MALDI imaging mass spectrometry for spatial mapping; shotgun lipidomics for rapid screening; confirm linkages with NMR of lipids.
Reference
Osawa T, Fujikawa K, Shimamoto K. Structures, functions, and syntheses of glycero-glycophospholipids. Front Chem. 2024;12:1353688. doi:10.3389/fchem.2024.1353688.
Wu T, Yin F, Guang S, He F, Yang L, Peng J. The glycosylphosphatidylinositol biosynthesis pathway in human diseases. Orphanet J Rare Dis. 2020;15(1):129. doi:10.1186/s13023-020-01401-z.
Müller GA, Müller TD. (Patho)physiology of glycosylphosphatidylinositol-anchored proteins I: localization at plasma membranes and extracellular compartments. Biomolecules. 2023;13(5):855. doi:10.3390/biom13050855.
Yokoyama N, Ekyalongo RC, Kage M, Hanafusa K, Nakayama H, Hirabayashi Y, Takamori K, Iwabuchi K. Phosphatidylglucoside regulates apoptosis of human neutrophilic lineage cells. Front Immunol. 2025;16:1597423. doi:10.3389/fimmu.2025.1597423.
Zhou K. Glycosylphosphatidylinositol-anchored proteins in Arabidopsis and one of their common roles in signaling transduction. Front Plant Sci. 2019;10:1022. doi:10.3389/fpls.2019.01022.
Hung HH, Nagatsuka Y, Soldà T, et al. Selective involvement of UGGT2 in protecting mouse embryonic fibroblasts from saturated lipid-induced ER stress. Proc Natl Acad Sci U S A. 2022;119(51):e2214957119. doi:10.1073/pnas.2214957119.
Almeida AM, Murakami Y, Baker A, et al. Targeted therapy for inherited GPI deficiency. N Engl J Med. 2007;356(16):1641-1647. doi:10.1056/NEJMoa063369.
Kinoshita T, Fujita M. Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J Lipid Res. 2016;57(1):6-24. doi:10.1194/jlr.R063313.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


