Integrative Proteomics and Metabolomics in Clinical Oncology: Unraveling Tumor Complexity
In clinical oncology, integrative proteomics and metabolomics—key pillars of multi-omics—are transforming cancer research by overcoming the limitations of genomic analysis.Cancer research faces significant challenges, including tumor heterogeneity, drug resistance, and difficulties in early diagnosis, which stem from the complex interplay of molecular aberrations and environmental factors. Genomic analyses alone often fail to fully characterize tumors, as many somatic mutations and copy number variants remain poorly understood, and patients frequently do not respond to therapies predicted by genomic profiles [1, 2]. This limitation underscores the need for multi-omics approaches, particularly proteomics and metabolomics, which provide deeper insights into tumor biology. Proteogenomics—the integration of proteomic and genomic data—offers a more complete view of cancer mechanisms by directly examining protein-level consequences of genomic aberrations, including post-translational modifications, and has already led to new biological and diagnostic knowledge [1, 2]. Similarly, metabolomics captures dynamic metabolic reprogramming in cancer cells, revealing alterations that drive tumor progression and therapy resistance. Together, these omics layers enable a holistic understanding of cancer pathogenesis, facilitating better patient stratification and therapeutic targeting [2, 3].
1. Proteomics in Cancer Research
Proteomics elucidates key regulatory mechanisms in cancer, such as epithelial-mesenchymal transition (EMT), dysregulated signaling pathways, and immune suppression, which cannot be fully discerned through genomic analysis alone [1]. For instance, proteomic studies reveal how tumor-associated macrophages (TAMs) are polarized by tumor-derived molecules to promote metastasis, highlighting the role of proteins in immune evasion [4, 5]. High-throughput quantitative methods, including data-independent acquisition (DIA) and parallel reaction monitoring (PRM) mass spectrometry, now allow for deep and quantitative characterization of tumor tissues. These technologies enable targeted proteomic measurements to be incorporated into clinical laboratories, supporting the advancement of clinical proteogenomics by providing robust data on protein expression and modifications [1, 2]. This approach not only identifies novel protein biomarkers but also uncovers mechanisms underlying therapy resistance, such as alterations in immune cell interactions within the tumor microenvironment [2, 5].
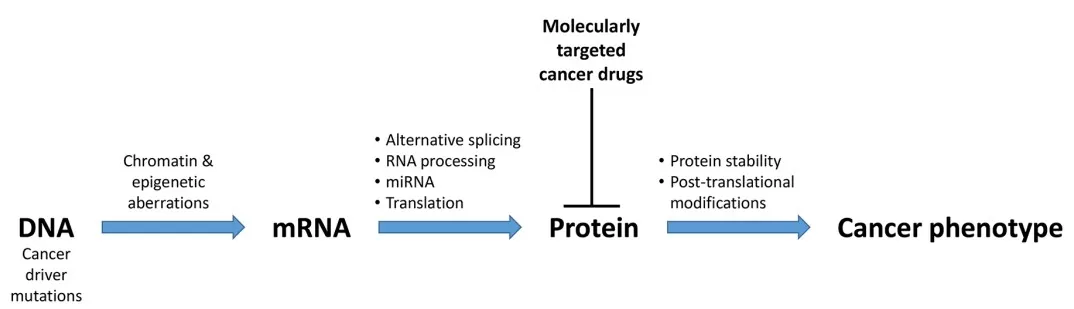
Many processes downstream of the genome affect the cancer phenotype
Recent clinical trials, such as those conducted by the Clinical Proteomic Tumor Analysis Consortium (CPTAC), have demonstrated how proteogenomics can uncover protein-level consequences of genomic alterations, leading to the identification of novel cancer biomarkers and therapeutic targets. Mass spectrometry-based proteomics, including DIA, PRM, and SWATH, is increasingly being integrated into clinical workflows for precision oncology.
2. Metabolomics in Oncology
Metabolomics focuses on the metabolic reprogramming inherent to cancer, where dysregulated pathways—such as lactate and glutamine metabolism—support tumor growth, invasion, and adaptation to microenvironments [3, 6]. Cancer cells harness metabolic alterations to obtain energy, membrane components, and signaling molecules, which are prominent in aggressive tumors like lung and liver cancer [6]. Untargeted metabolomics provides unbiased discovery of metabolic signatures, as seen in studies using mass spectrometry imaging to reveal spatial heterogeneity in metabolites associated with specific cell populations [7]. In early cancer detection, metabolomics has proven valuable for identifying oncometabolites—such as 2-hydroxyglutarate and succinate—that indicate metabolic dysregulation in tumor cells. These metabolites not only serve as biomarkers but also reveal therapeutic vulnerabilities within metabolic pathways.In contrast, targeted metabolomics, employing techniques like liquid chromatography-tandem mass spectrometry (LC-MS/MS), is ideal for validating specific biomarkers in clinical settings, such as identifying prognostic lipid species in colorectal cancer [8]. By integrating metabolomic profiling with proteomic data, researchers can validate metabolic biomarkers and better understand their functional roles in tumor progression.This dual approach allows researchers to map metabolic vulnerabilities, like nucleotide imbalances or urea cycle dysregulation, which can be exploited for therapeutic interventions [6].
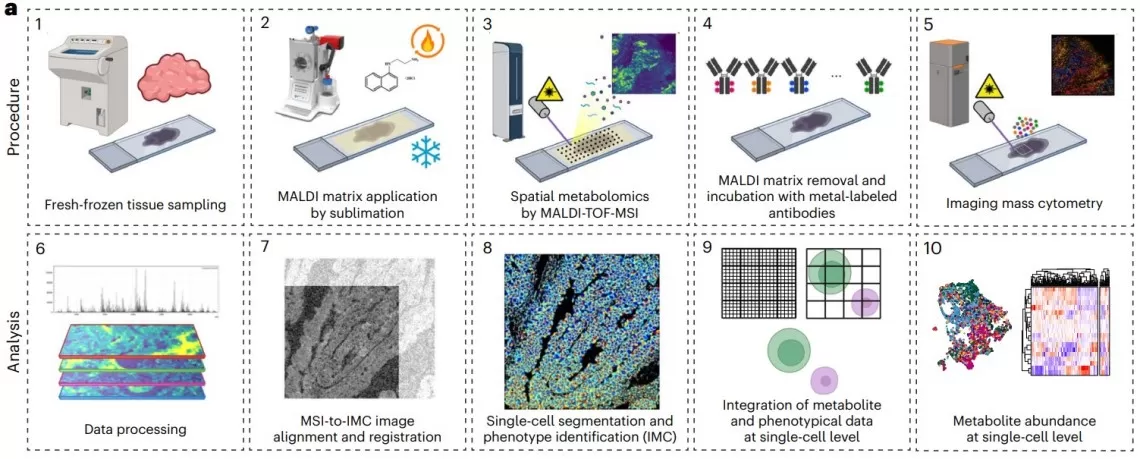
Integrated workflow of consecutive MALDI-MSI and IMC analyses
3. Why Multi-Omics in Oncology?
Integrating proteomics and metabolomics—as part of a broader multi-omics strategy—unveils complex regulatory networks and hierarchical interactions within tumors, overcoming the limitations of single-omics approaches. For example, combined proteomic and metabolomic data can reveal how mitochondrial-specific metabolic activation suppresses the tumor immune microenvironment, providing insights into mechanisms like oxidative phosphorylation in metastasis [6]. This integration is particularly effective for building predictive biomarker models, subtyping cancers based on molecular profiles, and forecasting therapeutic responses, as demonstrated by studies linking lipidomic signatures to disease-free survival and lymphovascular invasion in colorectal cancer [8]. Additionally, multi-omics facilitates the identification of synergistic targets, such as metabolic vulnerabilities in drug-resistant tumors, by correlating protein expression with metabolite fluxes [9]. AI-driven multi-omics analytics now enhance integration of proteomic and metabolomic datasets. Machine learning algorithms can identify complex biomarker patterns and predict patient responses to therapy, accelerating the translation of multi-omics research into clinical decision-making.A recommended visualization for this section is a multi-omics network diagram, illustrating how proteomic and metabolomic data converge to inform clinical decisions, such as patient stratification or treatment optimization [2, 10]. Overall, this holistic view enhances precision oncology by enabling more accurate prognostication and personalized therapy [1, 2].
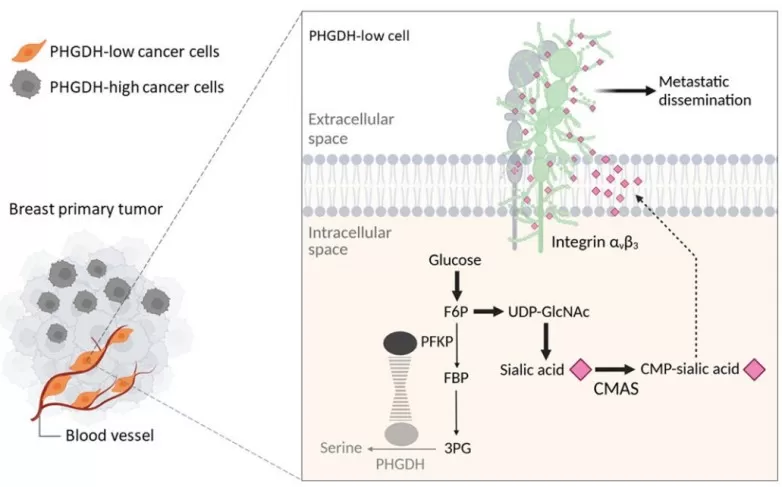
Schematic representation of the mechanism by which PHGDH-low cells increase metastatic dissemination from the primary tumor
4. Application Areas
Advancing Clinical Oncology Through Proteomics and Metabolomics: Insights from Key Research Applications Multi-omics approaches, particularly proteomics and metabolomics, are revolutionizing clinical oncology by enabling detailed molecular profiling that informs diagnosis, prognosis, and therapeutic strategies. Proteomics focuses on the comprehensive analysis of protein expression, modifications, and interactions, providing insights into signaling pathways and cellular mechanisms. Metabolomics, on the other hand, characterizes the dynamic repertoire of metabolites, revealing metabolic dysregulations associated with disease states. Together, these omics technologies enhance our understanding of tumor biology and facilitate precision medicine. Below, I summarize some papers, and elucidate how proteomics and metabolomics contributed to their key discoveries.
1) Tumor Metabolism & Microenvironment
Integrative proteomics and metabolomics map tumor metabolism–microenvironment interactions, revealing lipid pathways linked to aggressiveness.The article which is published by our partner clients, “Proto-oncogene Src links lipogenesis via lipin-1 to breast cancer malignancy”, investigates the mechanistic role of the Src proto-oncogene and lipin-1 in breast cancer malignancy, linking obesity-associated signaling to cancer progression [11]. The research employed human breast tumor samples and mouse models to address the obscure link between increased lipogenesis and poor cancer prognosis. Key findings show that obesity-related microenvironmental factors activate Src tyrosine kinase, which phosphorylates lipin-1 at Tyr398, Tyr413, and Tyr795 residues. This phosphorylation enhances lipin-1's phosphatidic acid phosphatase (PAP) activity, accelerating glycerophospholipid and triglyceride synthesis, and driving tumor growth and metastasis. For instance, mutation of these tyrosine residues (to phenylalanine) disabled lipin-1 from mediating Src-enhanced processes, inhibiting mammary tumor progression in mice. Critically, proteomics played a direct role in identifying the Src-lipin-1 interaction and phosphorylation sites, enabling detailed characterization of aberrant signaling that promotes malignancy. Metabolomics was integral to quantifying lipid metabolic shifts, such as glycerophospholipids and triglycerides, that underpin the observed tumor aggressiveness. Thus, integrating proteomic and metabolic data revealed novel molecular drivers of tumor metabolism heterogeneity, offering potential targets for therapeutic intervention.
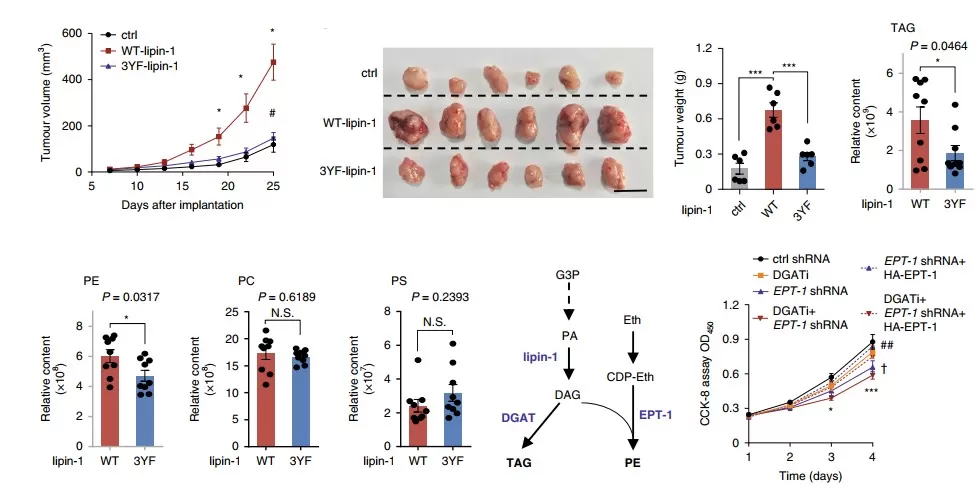
The proliferation of breast cancer cells depends on the tyrosine phosphorylation of lipin-1
2) Cancer Biomarkers & Early Detection
Multi-omics profiling discovers non-invasive biomarkers, improving diagnosis via metabolic and proteomic signatures.The article which is published by our partner clients, “Novel therapeutic evaluation biomarkers of lipid metabolism targets in uncomplicated pulmonary tuberculosis patients”, focused on pulmonary tuberculosis (TB) as a model for oncology-relevant biomarker discovery [12], and this research aimed to identify lipid-based biomarkers for early diagnosis and treatment efficacy. It used plasma samples from human TB patients monitored from diagnosis to cure to address the lack of potent medications and accurate biomarkers. Background highlights TB's reliance on host lipids as an energy source for ‘Mycobacterium tuberculosis’ (TB2, 2-month-treated TB patients; HC, health control). Main results revealed aberrant phospholipid, glyceride, and sphingolipid metabolism in patients; upon treatment, specific lipids like ceramide (Cer(d18:1/24:0)), LPA(0:0/16:0), and LPA(0:0/18:0) normalized, serving as potent biomarkers with 100% sensitivity and specificity for early diagnosis and therapeutic evaluation. Mechanistically, these metabolic changes could block ‘Mtb’ energy metabolism and autophagosome-lysosome fusion. Metabolomics was essential for this study, utilizing UPLC-MS/MS techniques to profile the plasma lipid spectrum and identify dysregulated metabolites such as ceramides and lysophosphatidic acids. Proteomics was less emphasized, but the correlation between lipid levels and clinical outcomes supports its integration for broader molecular biomarker validation. The metabolomic approach demonstrated high specificity, positioning lipid metabolites as promising non-invasive tools for oncology biomarker discovery in infectious diseases and cancer contexts.
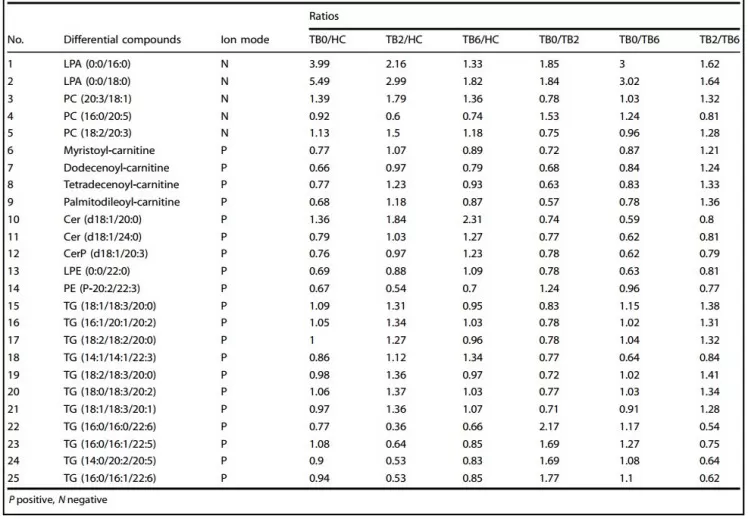
25 Differential lipid metabolites among TB0, TB2, TB6, and HC
3) Immunotherapy & Immune Response Prediction
Combining metabolomic biomarkers with proteomic immune profiling predicts immunotherapy response.This paper ‘Genome-wide characterization of circulating metabolic biomarkers’ applied multi-omics to characterize circulating metabolic biomarkers for immune-related prediction, aiming to uncover genetic determinants of systemic metabolism relevant to complex diseases like immune dysregulation [13]. Based on human cohorts from 33 studies involving up to 136,016 participants, it addressed the utility of genome-wide association studies (GWAS) in identifying metabolic traits for diagnostic insights. Key results identified over 400 independent loci associated with circulating metabolites (e.g., via nuclear magnetic resonance spectroscopy), allowing assignment of probable causal genes and highlighting genetic pleiotropy in metabolic pathways. Applications included characterizing intrahepatic cholestasis and suggesting causal links like that between acetone and hypertension. Metabolomics was central here, enabling the quantification of 233 metabolic traits to define biomarkers and associate them with genetic variation; proteomics was not explicitly detailed in the corpus but implicit in gene-to-protein pathway analysis. The study underscored metabolomics' role in immune response prediction by revealing metabolite signatures that could inform immunotherapy targets, such as inflammation-associated pathways, providing a resource for immune-oncology research and personalized therapeutic development.
 and non-fasted (bottom; total n = 58_1755141790_WNo_981d344.webp)
Results of the GWAS for glucose for the fasted (top; total n = 68,559) and non-fasted (bottom; total n = 58,112) cohorts
4) Drug Resistance Mechanisms
Proteomic–metabolomic integration reveals metabolic and protein interaction pathways behind therapy resistance.The article “Gut microbial carbohydrate metabolism contributes to insulin resistance” revealed exploring mechanisms underlying insulin resistance as a model for oncology drug resistance [14]. This research employed a multi-omics approach to link gut microbiota metabolism to metabolic disorders with implications for cancer risk. It analyzed human cohorts using unbiased fecal metabolomics, metagenomics, host metabolomics, and transcriptomics to address the unclear role of microbiome in pathology. Findings showed that gut bacterial carbohydrate metabolism elevates host-accessible monosaccharides in insulin-resistant individuals, associating with microbial pathways, inflammatory cytokines, and insulin sensitivity. Specific bacteria were identified that ameliorate insulin resistance in mouse models, suggesting therapeutic targets for obesity-related diseases. Metabolomics was crucial for profiling fecal carbohydrates and host metabolites, enabling the detection of dysregulated energy metabolism (e.g., contributions to 10% of host energy extraction). Proteomics was inferenced in transcriptomic data for bacterial enzyme expression, providing insights into pathways like carbohydrate metabolisms that drive resistance mechanisms. This multi-omics integration revealed how microbiota-derived metabolism influences host responses, offering a model for understanding metabolic drivers of drug resistance in cancers, such as those linked to immune evasion.
5) Cancer Metastasis & Prognostic Stratification
Correlating protein expression with metabolic alterations stratifies metastatic risk.The article “PHGDH heterogeneity potentiates cancer cell dissemination and metastasis” investigated the role of phosphoglycerate dehydrogenase (PHGDH) heterogeneity in cancer metastasis and prognosis, focusing on breast cancer models. It analyzed human patient tissues and mouse xenografts to determine how metabolic heterogeneity contributes to dissemination [9], addressing its understudied impact. Results showed that low or heterogeneous PHGDH expression correlates with poor metastasis-free survival in breast cancer patients, and silencing ‘Phgdh’ in mice increased metastasis by promoting integrin sialylation through hexosamine-sialic acid pathway activation. Mechanistically, this led to aberrant protein glycosylation, enhancing cell migration and invasion, with PHGDH heterogeneity serving as a sign of tumor aggressiveness. Proteomics was fundamental to this discovery, identifying the PHGDH-phosphofructokinase interaction and glycosylation modifications (e.g., sialylation of integrin αβ), which were key to metastasis promotion. Metabolomics supported this by revealing shifts in glycosylation precursor pathways, such as hexosamine-sialic acid metabolism. Thus, combining proteomic and metabolic analyses elucidated non-catalytic mechanisms that stratify metastatic risk, enabling prognostic biomarker development and targeted therapies.
In conclusion, these papers exemplify how proteomics and metabolomics synergize to advance clinical oncology across diverse applications, from elucidating metabolic vulnerabilities to identifying non-invasive biomarkers. The integrated multi-omics approaches enhance mechanistic understanding, predict treatment responses, and stratify patient risk, ultimately paving the way for personalized oncology strategies. By adopting integrative proteomics and metabolomics in oncology, researchers and clinicians can achieve unprecedented precision in cancer diagnosis, prognosis, and treatment design, driving the next generation of precision medicine.
At MetwareBio, we provide one-stop proteomics, metabolomics, lipidomics, and multi-omics services to support your cancer research. From biomarker discovery to mechanistic studies, our advanced mass spectrometry platforms and bioinformatics expertise help you generate high-quality data for impactful clinical and translational outcomes.
FAQs
Q1: What proteomic technologies are most used in clinical oncology?
DIA, PRM, SWATH and other MS-based approaches quantify proteins, PTMs, and interactions for clinically actionable insights.
Q2: What is proteogenomics in cancer research?
Proteogenomics combines proteomic and genomic data to reveal functional consequences of genetic alterations.
Q3: How does metabolomics aid in early cancer detection?
Identifies disease-specific metabolic changes, including oncometabolites, for early diagnosis.
Q4: Why integrate proteomics and metabolomics for biomarker discovery?
Offers a comprehensive view of tumor biology, yielding robust, clinically actionable biomarkers.
References
1. Zhang, B., et al., Clinical potential of mass spectrometry-based proteogenomics. Nat Rev Clin Oncol, 2019. 16(4): p. 256-268.
2. Mani, D.R., et al., Cancer proteogenomics: current impact and future prospects. Nat Rev Cancer, 2022. 22(5): p. 298-313.
3. Bian, X., et al., Lipid metabolism and cancer. J Exp Med, 2021. 218(1).
4. Wu, J.Y., et al., Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol Cell, 2020. 77(2): p. 213-227.e5.
5. Zhang, J., et al., Multi-omics analysis reveals the chemoresistance mechanism of proliferating tissue-resident macrophages in PDAC via metabolic adaptation. Cell Rep, 2023. 42(6): p. 112620.
6. Parker, A.L., et al., Creatine riboside is a cancer cell-derived metabolite associated with arginine auxotrophy. J Clin Invest, 2022. 132(14).
7. Nunes, J.B., et al., Integration of mass cytometry and mass spectrometry imaging for spatially resolved single-cell metabolic profiling. Nat Methods, 2024. 21(10): p. 1796-1800.
8. Ecker, J., et al., The Colorectal Cancer Lipidome: Identification of a Robust Tumor-Specific Lipid Species Signature. Gastroenterology, 2021. 161(3): p. 910-923.e19.
9. Rossi, M., et al., PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature, 2022. 605(7911): p. 747-753.
10. Ravi, V.M., et al., Spatially resolved multi-omics deciphers bidirectional tumor-host interdependence in glioblastoma. Cancer Cell, 2022. 40(6): p. 639-655.e13.
11. Song, L., et al., Proto-oncogene Src links lipogenesis via lipin-1 to breast cancer malignancy. Nat Commun, 2020. 11(1): p. 5842.
12. Chen, J.X., et al., Novel therapeutic evaluation biomarkers of lipid metabolism targets in uncomplicated pulmonary tuberculosis patients. Signal Transduct Target Ther, 2021. 6(1): p. 22.
13. Karjalainen, M.K., et al., Genome-wide characterization of circulating metabolic biomarkers. Nature, 2024. 628(8006): p. 130-138.
14. Takeuchi, T., et al., Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature, 2023. 621(7978): p. 389-395.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


