Parallel Reaction Monitoring (PRM) in Mass Spectrometry: A High-Resolution Targeted Quantification Method
Parallel Reaction Monitoring (PRM) is a high-resolution, high-specificity, and high-accuracy targeted quantification technique widely used in proteomics, metabolomics, and biomarker validation. Unlike data-dependent acquisition (DDA) or data-independent acquisition (DIA), PRM focuses on a predefined list of analytes, providing precise quantitative data with minimal interference. This blog delves into the principles of PRM, how it compares with SRM/MRM, its workflow, application scenarios, and why PRM is a go-to method for quantitative mass spectrometry.
What Is PRM (Parallel Reaction Monitoring)?
PRM is a targeted mass spectrometry technique that combines the selectivity of SRM/MRM with the power of high-resolution, accurate-mass (HRAM) analyzers like Orbitrap or Q-TOF. It involves the isolation of specific precursor ions, fragmentation in the collision cell, and the collection of full MS/MS spectra of the resulting product ions.
Key Features:
- Uses a quadrupole to isolate the target precursor ion (m/z).
- Performs high-energy collisional dissociation (HCD) to generate product ions.
- Detects all fragment ions in parallel using a high-resolution detector (e.g., Orbitrap).
- Enables post-acquisition data extraction of fragment ions using software like Skyline.
This approach enhances analytical sensitivity, improves quantitative reproducibility, and simplifies method development for complex biological samples.
 mass spectrometry _1750152326_WNo_733d476.webp)
Schematic representation of parallel reaction monitoring (PRM) mass spectrometry (Bezstarosti et al., 2024)
PRM vs SRM/MRM: What’s the Difference?
Selected Reaction Monitoring (SRM), also known as Multiple Reaction Monitoring (MRM), has traditionally served as the benchmark method for targeted quantification using triple quadrupole mass spectrometers. This technique relies on monitoring specific precursor-to-fragment ion transitions with high sensitivity, making it a reliable choice for quantitative proteomics, metabolite analysis, and clinical assay development. However, SRM methods require careful optimization of each transition, including collision energy and dwell time, and they operate at unit resolution, which can result in signal interference from co-eluting or isobaric compounds in complex matrices.
In contrast, Parallel Reaction Monitoring (PRM) offers distinct advantages by utilizing high-resolution, accurate-mass (HRAM) instruments like Orbitrap or Q-TOF. PRM acquires full MS/MS spectra for each selected precursor, enabling simultaneous monitoring of all fragment ions without the need for predefined transitions. This improves method flexibility, reduces setup time, and enhances selectivity by resolving interfering species with greater mass accuracy. As a result, PRM is increasingly favored in biomarker validation, targeted proteomics, and clinical metabolomics workflows where both precision and robustness are essential.
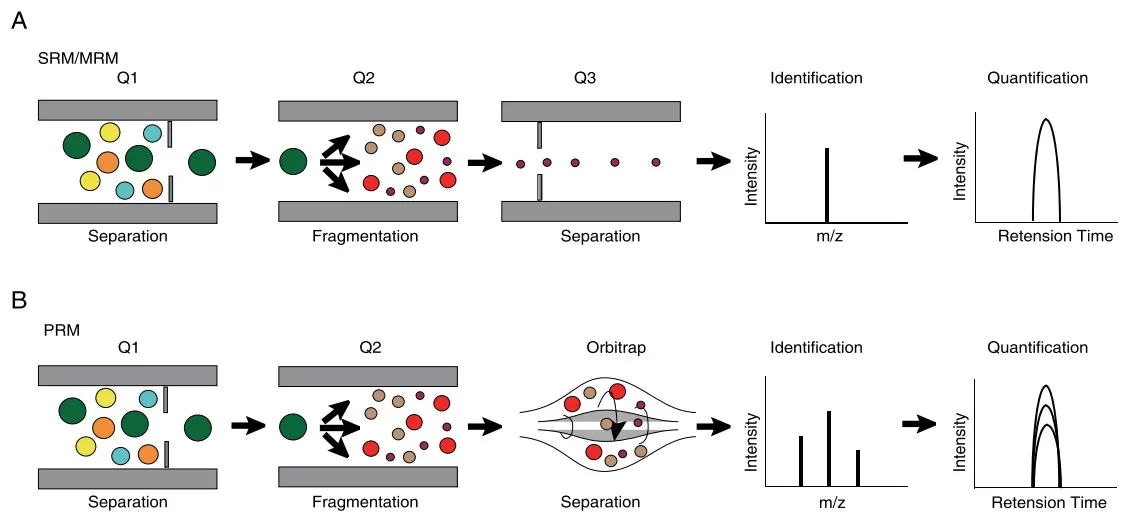
The working princeples of PRM and SRM/MRM (Lan et al., 2021)
To better illustrate the key differences between these two targeted mass spectrometry strategies, we’ve provided a comparison table below.
PRM vs SRM/MRM — Instrumentation and Method Comparison
|
Feature |
PRM (Parallel Reaction Monitoring) |
SRM/MRM |
|
Instrument |
Orbitrap, Q-TOF (HRAM) |
Triple quadrupole |
|
Mass Resolution |
High (≥30,000 FWHM) |
Low (unit resolution) |
|
Data Acquisition |
Full MS2 spectra per precursor |
Single transition (precursor > fragment) |
|
Transition Selection |
All fragment ions |
Predefined fragment ions only |
|
Method Development |
Simple, minimal optimization |
Complex, requires tuning |
|
Retrospective Analysis |
Yes |
No |
|
Data Processing |
Skyline, Xcalibur |
Skyline, MultiQuant |
Advantages of PRM in Quantitative Proteomics & Metabolomics
1. Superior Selectivity and Specificity
PRM leverages high-resolution, accurate-mass (HRAM) detectors such as Orbitrap to distinguish target analytes from chemically similar background ions. This significantly reduces matrix interferences and chemical noise, which is critical when analyzing low-abundance peptides or metabolites in complex biological samples. The enhanced selectivity improves confidence in both identification and quantification, particularly in targeted proteomics and clinical metabolomics studies.
2. Simplified Method Development
Unlike SRM/MRM, which requires time-consuming optimization of individual transitions, PRM captures full MS/MS spectra for each precursor without predefined fragment ion selection. This eliminates the need for extensive tuning and simplifies experimental setup, making PRM highly scalable for multiplexed assays. As a result, researchers can develop targeted quantification methods more quickly while maintaining analytical robustness.
3. Improved Quantitative Accuracy
By acquiring comprehensive MS2 spectra, PRM allows for precise peak integration across multiple fragment ions, enhancing quantification reliability. This approach reduces susceptibility to co-eluting interferences and improves linearity across a wide dynamic range, which is essential for accurately measuring biomolecules in samples with variable concentrations. These features make PRM well-suited for quantitative mass spectrometry in both discovery validation and clinical research.
4. Versatility in Quantification Strategies
PRM supports both label-free quantification and workflows using stable isotope-labeled internal standards, giving researchers flexibility to choose between relative or absolute quantification. This makes it adaptable to a broad range of study designs, from exploratory proteomics to regulated biomarker verification. Its compatibility with isotope dilution strategies also enhances accuracy in absolute quantification of peptides and metabolites.
5. Retrospective Data Mining
One of the most powerful advantages of PRM is its ability to store complete fragment ion data, enabling post-acquisition reanalysis. Researchers can revisit the raw data to quantify additional targets or verify results without re-running the sample. This feature enhances data utility and reproducibility, which is especially valuable in long-term projects, clinical validation studies, or when updating target panels based on new discoveries.
PRM Workflow: From Sample to Quantification
1. Sample Preparation
In PRM-based workflows, sample preparation is the foundation for achieving accurate and reproducible results. In proteomics, this typically involves extracting proteins from biological matrices such as plasma, tissue, or cultured cells, followed by enzymatic digestion—most commonly with trypsin—to produce peptides suitable for mass spectrometry analysis. In targeted metabolomics, small molecule metabolites are extracted using organic solvents like methanol or acetonitrile, often under cold conditions to preserve stability. The use of internal standards at this stage helps normalize variability and improve downstream quantitation accuracy.
2. Target Selection and Inclusion List Creation
Once the sample is prepared, specific analytes must be selected for targeted analysis. Precursor ions—representing peptides or metabolites of interest—are chosen based on prior DDA (data-dependent acquisition) experiments, public databases, or established spectral libraries. Each selected precursor is defined by its m/z, charge state, and expected retention time. These parameters are compiled into an inclusion list that instructs the instrument which ions to isolate and monitor. Properly curated target lists enhance specificity, reduce ambiguity, and ensure that the PRM experiment focuses on the most relevant biological markers.
3. Instrument Setup
Configuring the mass spectrometer accurately is crucial for optimal PRM performance. Common settings include selecting an isolation window between 0.4 and 2.0 Da, setting Orbitrap resolution (typically 30,000 to 120,000 FWHM), optimizing collision energy for efficient fragmentation, and scheduling retention time windows to increase throughput. These parameters must be tuned to balance sensitivity, cycle time, and data quality, especially when analyzing complex samples with multiple targets. Proper setup ensures efficient acquisition of high-quality MS/MS data for each selected precursor.
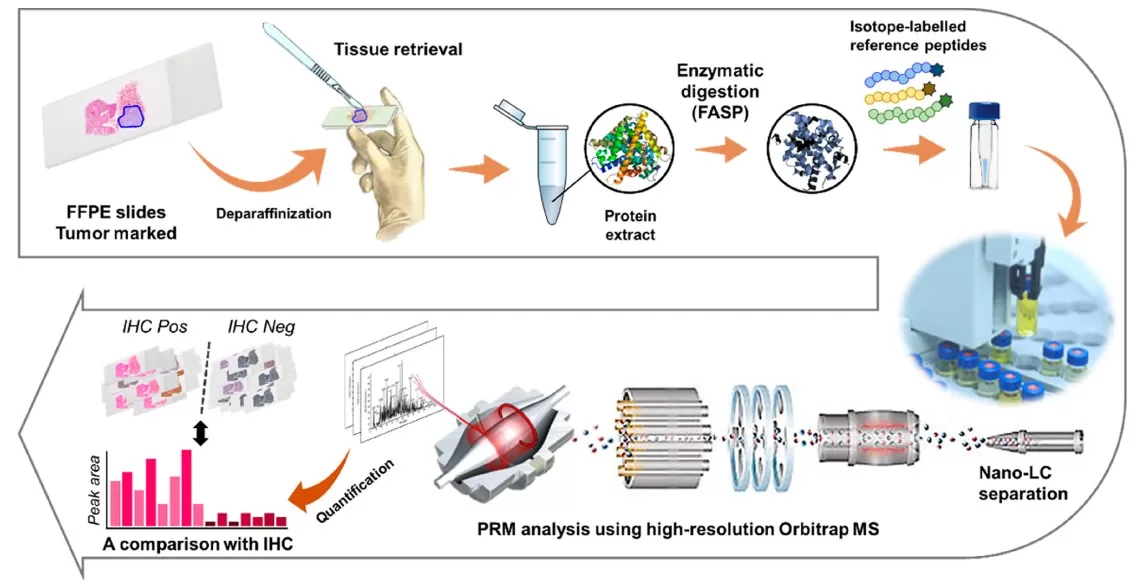
PRM workflow using FFPE tissue slides (Park et al., 2020)
4. Data Acquisition
During data acquisition, the instrument isolates each precursor ion in Q1, fragments it using HCD (Higher-energy Collisional Dissociation), and records the full spectrum of resulting fragment ions using a high-resolution mass analyzer such as Orbitrap or Q-TOF. This allows simultaneous detection of all product ions in parallel, rather than requiring pre-selection of specific transitions. As a result, PRM provides a complete MS/MS profile for each target, improving both identification confidence and quantification reliability.
5. Data Analysis
The final step involves processing the acquired data using software like Skyline, which enables targeted extraction of fragment ion chromatograms from the full MS/MS datasets. Researchers can verify co-elution of multiple fragment ions, integrate peak areas, correct for background interference, and normalize signal intensities using internal standards. Skyline also supports statistical analysis and customizable reports, making it a comprehensive tool for PRM data interpretation. This combination of robust acquisition and rigorous analysis ensures high-quality, quantitative insights into target peptides or metabolites.
PRM Applications in Life Science Research
1. Targeted Proteomics
In the field of targeted proteomics, PRM offers a highly specific and reproducible method for quantifying predefined proteins in complex biological samples such as plasma, cerebrospinal fluid, or tissue lysates. It is particularly valuable for validating biomarkers discovered in large-scale discovery proteomics studies, allowing researchers to verify differential protein expression with high confidence. PRM is also well-suited for pathway-centric studies, where specific sets of proteins related to signaling cascades, metabolism, or disease mechanisms need to be precisely monitored. Its high-resolution capability reduces interference and enhances quantification accuracy, making it a gold-standard tool in translational proteomics.
2. Clinical Metabolomics
PRM is increasingly applied in clinical metabolomics for the precise quantification of small molecules such as amino acids, organic acids, neurotransmitters, and lipid mediators. These metabolites are often present at low concentrations and require high-resolution detection to distinguish them from structurally similar compounds. PRM enables selective monitoring of target metabolites in complex matrices like serum, urine, or tissue extracts, which is essential for disease diagnostics, treatment monitoring, and metabolic profiling. Its compatibility with stable isotope-labeled standards further enhances its use in absolute quantification of clinically relevant biomarkers.
3. Pharmacokinetics and Toxicology
In pharmacokinetic (PK) and toxicology studies, PRM provides a sensitive and specific approach for tracking drug candidates and their metabolites over time. It enables researchers to monitor absorption, distribution, metabolism, and excretion (ADME) parameters with precision, facilitating dose-response assessments and toxicokinetic evaluations. Because PRM can quantify both parent compounds and their biotransformation products in a single run, it is especially useful in drug development pipelines where detailed metabolic insights are critical for regulatory submission and safety evaluation.
4. Post-Translational Modification (PTM) Studies
PRM is particularly effective for analyzing post-translational modifications (PTMs) such as phosphorylation, acetylation, methylation, and ubiquitination. These modifications often play critical roles in cellular regulation and disease pathogenesis, but detecting them requires high mass accuracy and selectivity due to their low abundance and isobaric interferences. PRM enables site-specific monitoring of modified peptides with exceptional resolution, allowing researchers to quantify dynamic PTM changes across different biological conditions or treatments. This makes it a powerful tool in epigenetics and cell signaling research.
5. Food Safety and Environmental Monitoring
Beyond biomedical research, PRM has applications in food safety and environmental monitoring, where the detection of trace-level contaminants, residues, or toxins is vital. Whether it's quantifying pesticide residues in agricultural products, monitoring mycotoxins in foodstuffs, or detecting pharmaceutical pollutants in water sources, PRM offers high-resolution quantification with minimal false positives. Its ability to perform retrospective analysis also makes it ideal for confirming the presence of new or emerging contaminants without the need for re-acquisition.
Reference
Bezstarosti, K., Van der Wal, L., Doff, W. A. S., & Demmers, J. A. A. (2024). Parallel reaction monitoring targeted mass spectrometry as a fast and sensitive alternative to antibody-based protein detection. Frontiers in Analytical Science, 4. https://doi.org/10.3389/frans.2024.1397810
Lan, Y., Zeng, X., Xiao, J., Hu, L., Tan, L., Liang, M., Wang, X., Lu, S., Long, F., & Peng, T. (2021). New advances in quantitative proteomics research and current applications in asthma. Expert review of proteomics, 18(12), 1045–1057. https://doi.org/10.1080/14789450.2021.2017777
Park, J., Oh, H. J., Han, D., Wang, J. I., Park, I. A., Ryu, H. S., & Kim, Y. (2020). Parallel Reaction Monitoring-Mass Spectrometry (PRM-MS)-Based Targeted Proteomic Surrogates for Intrinsic Subtypes in Breast Cancer: Comparative Analysis with Immunohistochemical Phenotypes. Journal of proteome research, 19(7), 2643–2653. https://doi.org/10.1021/acs.jproteome.9b00490
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


