Biological Waxes: Composition, Plant Cuticular Waxes, and GC-MS Profiling
Biological waxes are hydrophobic lipid layers found in plants, animals, and microbes; in plants, they form cuticular waxes that coat aerial organs and deliver drought/heat protection, UV shielding, and pathogen/insect defense. These plant wax mixtures are composed of long-chain lipids that contribute to drought resistance, fruit quality preservation, and defense against pests. Their composition and biosynthesis are central to both fundamental plant biology and applied agricultural research. In this article, we explore the molecular nature of plant waxes, how they function in environmental adaptation, and how GC-MS and GC×GC-MS profiling techniques are used to analyze them. These insights support advancements in crop breeding, stress tolerance, and postharvest management.

Figure 1. glaucous vs glossy wheat epicuticular wax bloom
Source: Wang Y et al. “Molecular Mapping of the W1 Locus Controlling Glaucousness in Bread Wheat,” PLOS ONE (2015) Fig. 1. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
1. What Are Waxes in Biology? (Definition & Scope)
In biology, waxes occur in plants, animals, and microbes as long-chain hydrophobic lipids. Here we focus on plant cuticular wax— the solvent-extractable layer over cutin—while noting cross-kingdom signatures where relevant. In plants, cuticular wax refers to the solvent-extractable lipids forming the outermost layer of the cuticle—a protective barrier covering leaves, stems, flowers, and fruits. Unlike structural cutin, which is a polyester matrix, waxes are unbound and form the visible epicuticular film or crystalline layer. These waxes include alkanes, alcohols, aldehydes, ketones, and triterpenoids, and are essential to the plant’s interaction with its environment. Notably, plant waxes played a key role in the evolutionary transition from aquatic to terrestrial life by reducing desiccation. Today, they remain vital for survival under drought, UV radiation, and pathogen pressure.
2. Core Functions of Cuticular Waxes
|
Function |
Primary Mechanism |
|
Reduce non-stomatal water loss |
Hydrophobic barrier; longer-chain alkanes (C27–C33) lower cuticular conductance |
|
UV/heat mitigation |
Increased reflectance; wax bloom (β-diketones) cools leaf surface |
|
Pathogen & insect exclusion |
Surface roughness and adverse chemistry reduce adhesion/oviposition |
|
Self-cleaning |
Micro/nano crystals enable lotus effect (high contact angle) |
|
Postharvest firmness |
Wax esters & triterpenes limit transpiration, preserve turgor |
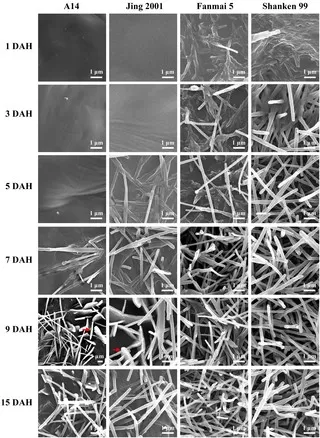
Figure 2. sem evolution wax crystals wheat glumes
Source: Wang Y et al., PLOS ONE (2015) Fig. 6. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
3. Chemical Composition of Biological Waxes
Biological waxes (natural waxes) are mixtures of long-chain, hydrophobic lipids that build protective, water-repellent barriers across kingdoms. They are dominated by very-long-chain (VLC) aliphatics and wax esters (a long-chain fatty acid esterified to a long-chain fatty alcohol), with minor oxygenated derivatives that tune crystallinity and melting behavior.
Core chemical families (cross-kingdom):
- n-Alkanes (often very long chain), plus branched (iso/anteiso) homologs that adjust packing.
- Primary/secondary long-chain alcohols and fatty aldehydes.
- Fatty acids, ketones, and β-diketones (prominent in some cereal cuticles; linked to glaucous wax bloom).
- Wax esters (fatty acid + fatty alcohol) as major structural or storage lipids.
- Triterpenoids/sterols and their esters (important in certain animal and plant waxes).
- Hydroxy esters/diesters present at lower abundance in specific taxa.
Kingdom-specific signatures:
- Plants (plant cuticular / epicuticular wax): enriched in n-alkanes (often C27–C33), primary alcohols, aldehydes, ketones, and β-diketones that produce a glaucous wax bloom; many fruit skins contain free triterpenoids (e.g., ursolic/oleanolic acids) and wax esters influencing gloss and firmness.
- Animals (animal waxes): beeswax is predominantly long-chain wax esters with hydrocarbons and minor free acids/alcohols; lanolin (wool wax) is rich in sterol esters and hydroxy components that confer plasticity/emollience; marine waxes (e.g., spermaceti) are high in wax esters used for buoyancy/energy storage.
- Microbes & algae (microbial wax esters): certain bacteria and microalgae synthesize storage wax esters under carbon-excess conditions, relevant to bio-based materials.
Key point: composition is species-, tissue-, and environment-dependent. This chemical diversity underpins functional traits—from reduced non-stomatal water loss and UV/heat mitigation in plants to barrier/emollient roles in animal waxes and energy storage in marine/microbial systems.
4. Biosynthesis and Regulation of Plant Waxes
Plant waxes are synthesized in epidermal cells through a tightly regulated pathway. The process starts with basic fatty acids, which are gradually extended into longer chains and then converted into different wax compounds.
Pathway snapshot. VLC acyl chains are elongated by the FAE complex (KCS/CER6, KCR, HCD, ECR/CER10). They branch into:
- Alcohol/ester branch: FARs generate primary alcohols; WSD1 (wax synthase) forms wax esters.
- Alkane-forming branch: CER1–CER3 (with CYTB5) produce alkanes, which can be oxidized to secondary alcohols/ketones.
Cyclic triterpenoids originate from isoprenoid metabolism via oxidosqualene cyclases (OSC).
Export and assembly. ABCG12 (CER5) and ABCG11 half-transporters, together with LTP/LTPG proteins, mediate movement to the cuticle, where molecules self-assemble into films and crystals.
Regulation. Drought, high light, and ABA up-regulate MYB94/MYB96/WIN1 and downstream wax genes, increasing total load and altering chain-length distribution and class balance.
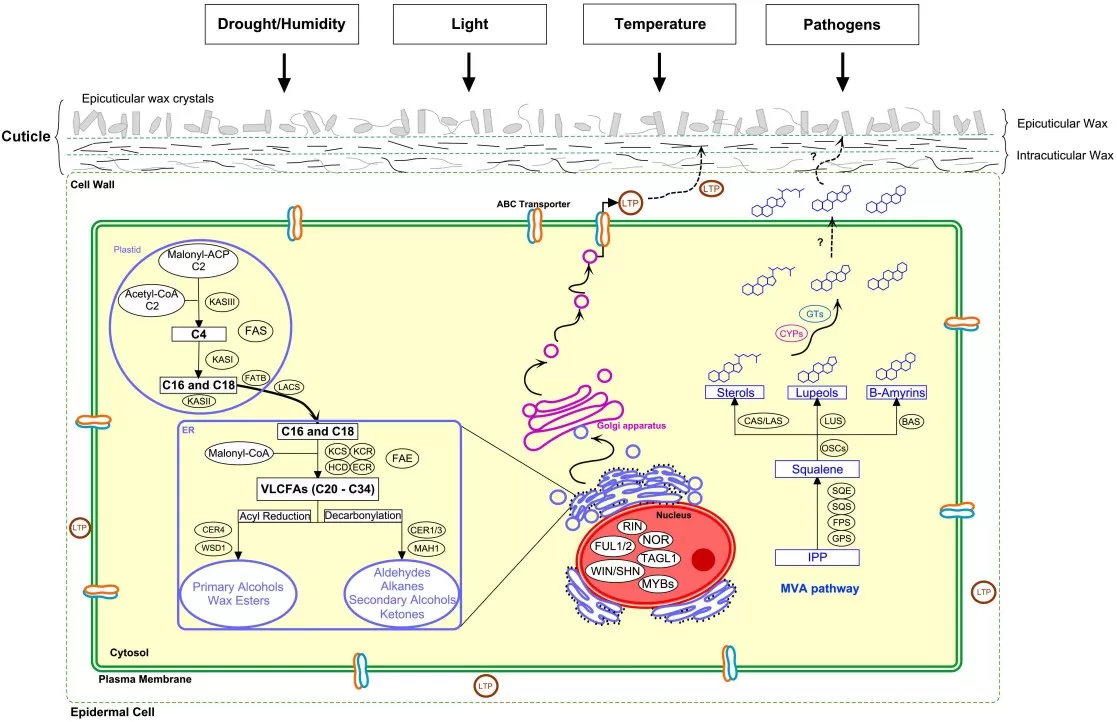
Figure 3. cuticular wax biosynthesis and regulation schematic
Source: Trivedi P et al. “Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits,” Frontiers in Plant Science (2019) Fig. 1. CC BY 4.0. No endorsement implied.
5. Sampling and Extraction for Wax Profiling
Accurate wax analysis begins with careful sampling and clean extraction techniques to isolate surface lipids without internal contamination.
Sample selection: Use dry, undamaged plant surfaces like leaves, fruit skins, or stems. Measure surface area or weight to enable normalization.
Solvent washing: Briefly dip or rinse samples (10–30 seconds) in a non-polar solvent such as hexane or chloroform to dissolve surface waxes only.
Wax collection: Transfer the extract to a clean vial. A short second wash may improve yield. Avoid prolonged exposure to prevent extracting unrelated compounds.
Internal standard: Add a known lipid for normalization during downstream quantification.
Evaporation: Remove the solvent gently using nitrogen or rotary evaporation to isolate the wax fraction.
Derivatization (if needed): Treat with a silylating agent to improve GC detection of alcohols or acids.
Quality control & normalization. Report wax amounts per leaf area (cm²) or per fruit surface; use leaf discs or image-based area estimates. Include a long-chain internal standard (e.g., C32 n-alkane) added before extraction.
These steps provide a reproducible and surface-specific profile of cuticular wax composition, forming a reliable basis for downstream GC-MS analysis.
Common pitfalls. Over-long solvent dips extract intracuticular/cutin components; rubbing damages crystals; wet surfaces dilute analyte. Keep dip 10–30 s, work on dry tissue, standardize temperature/time, and run procedural blanks.
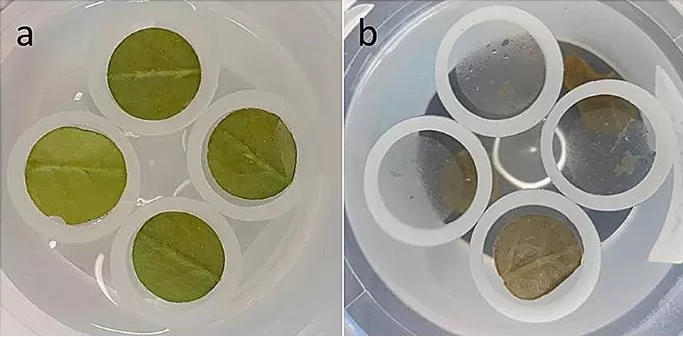
Figure 4. workflow enzymatic isolation leaf cuticle arabidopsis
Source: Vráblová M et al. “A modified method for enzymatic isolation…,” Plant Methods (2020) Fig. 1. CC BY 4.0. No endorsement implied.
6. GC-MS and GC×GC-MS Workflow for Wax Analysis
Gas chromatography–mass spectrometry (GC-MS) is the gold standard for identifying and quantifying plant wax components. It separates and detects complex mixtures with high sensitivity.
Sample injection: The wax extract (often derivatized) is injected into the GC system.
Separation (GC): Compounds are vaporized and pass through a non-polar column. They separate based on volatility and polarity, producing distinct peaks. Figure 5 shows a representative 1D GC–MS total ion chromatogram (TIC) of plant cuticular wax extracts, illustrating well-resolved peaks for major alkanes/alcohols.
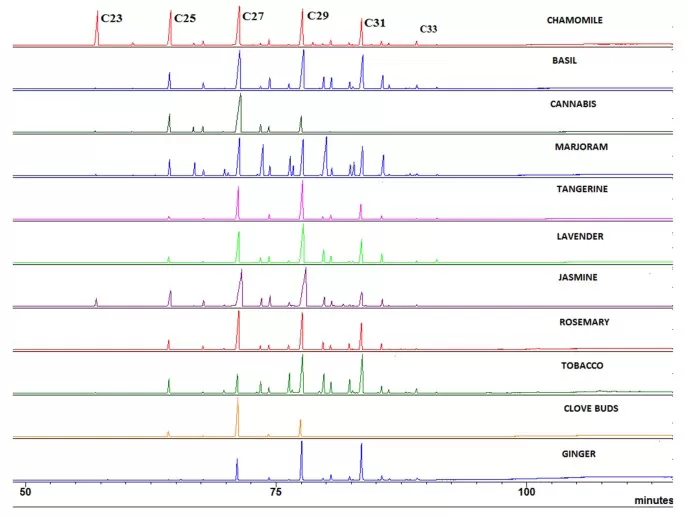
Figure 5. gcms traces cuticular waxes from multiple plants
Source: Scognamiglio M et al. “Fractional Separation and Characterization of Cuticular Waxes…,” Separations (MDPI) 2022, Fig. 2. CC BY 4.0. No endorsement implied.
Detection (MS): As each compound exits the column, the MS unit ionizes and fragments it. The resulting mass spectra are compared with reference libraries to identify each component. Additionally, report Kovats retention indices (RI) and match EI (70 eV) spectra to NIST/Wiley libraries, confirming key compounds with authentic standards.
Quantification: The internal standard allows for accurate calculation of wax content, often reported as µg cm⁻² (leaves) or µg g⁻¹ FW (fruits).
For highly complex samples, GC×GC-MS offers enhanced resolution. It uses two columns of different properties and a modulator to achieve two-dimensional separation. This allows clear identification of overlapping or trace-level compounds that 1D GC might miss. Figure 6 depicts a GC×GC contour plot highlighting two-dimensional separation that resolves coeluting or trace-level components.
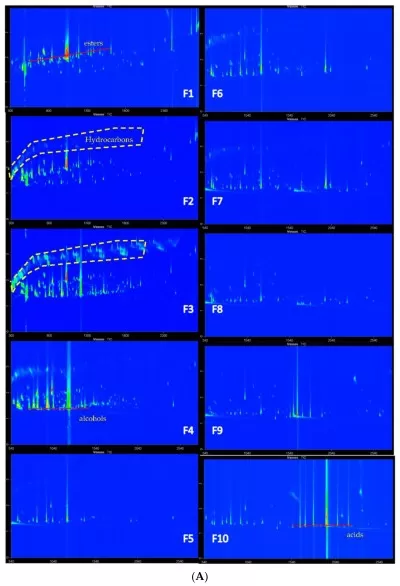
Figure 6. gcxgc contour plot fraction series fig3A
Source:Zheng J, He Z, Yang K, Liu Z, Zhao D, Qian MC. Volatile Analysis of Wuliangye Baijiu by LiChrolut EN SPE Fractionation Coupled with Comprehensive GC×GC-TOFMS. Molecules. 2022;27(4):1318. Figure 3A. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Together, GC-MS and GC×GC-MS enable detailed cuticular wax profiling critical for plant biology, stress physiology, and metabolomics research.
7. Wax-Mediated Drought & Heat Stress Resilience
Among biological waxes, plant cuticular wax is pivotal for drought and heat resilience, with higher wax load—especially long-chain alkanes—consistently linked to reduced non-stomatal water loss and cooler leaf surface temperature. In rice, loss of the wax-regulating gene OsMYB60 produced a thinner cuticle, faster water loss, and heightened drought sensitivity (2022, Plant J). In cotton, silencing GhFAR3.1 impaired wax accumulation and reduced survival under water deficit (2021, BMC Plant Biol). Across cereals with a visible epicuticular wax bloom (the glaucous, whitish layer enriched in β-diketones), leaves often run several degrees cooler in full sun, a practical advantage in arid climates. Together, these lines of evidence underscore wax load and composition as integral traits for drought and heat resilience.
8. Wax-Enhanced Postharvest Fruit Quality & Chilling Injury
Within the broader family of biological waxes, cuticular wax esters and triterpenes in fruit skins directly shape postharvest outcomes by slowing transpiration and texture loss, thereby lowering chilling injury risk. Blueberries with higher triterpene levels lost less water and retained firmness during storage (2023, Foods). Preserving cuticle integrity lowered chilling injury and slowed softening in cold-stored plums (2024, Sci Hortic), with similar protective trends observed in tropical fruit such as papaya. In commercial practice, edible coatings often reinforce the native wax barrier without altering sensory quality when properly formulated, highlighting the central role of epicuticular wax in maintaining fruit marketability under refrigeration.
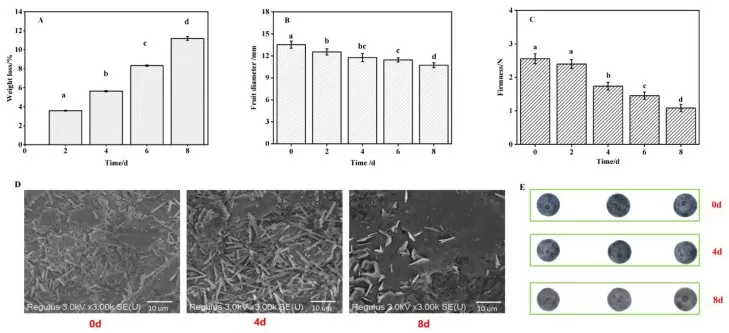
Figure 7. blueberry cuticular wax triterpenes storage quality
Source: Kong Q et al. “Cuticular Wax Triterpenes Maintain Storage Quality of Blueberries…,” Foods (MDPI) 2023, Fig. 2. CC BY 4.0. No endorsement implied.
9. Wax-Based Insect & Herbivore Resistance
As a biological wax barrier, epicuticular crystals and surface chemistry hinder insect landing, adhesion, and oviposition, providing a passive first-line defense that complements endogenous toxins. Cabbage with a pronounced epicuticular wax bloom deterred Pieris rapae feeding and egg-laying (2025, J Chem Ecol), and cotton engineered for elevated cuticular wax exhibited fewer whiteflies and reduced virus incidence (2021, PLoS One). These findings position wax bloom properties—including β-diketone-rich glaucousness—as ecologically meaningful features of plant–insect interactions.
10. Wax Traits for Bioenergy & Climate Resilience
In bioenergy crops, heavy plant cuticular wax loads—one branch of biological wax diversity—help stabilize water status and canopy temperature on marginal lands under climatic extremes. In sorghum, wax accumulation was associated with improved drought and heat tolerance (2023, Front Plant Sci). Beyond resilience, surface wax can represent a coproduct opportunity—estimates of ~90–180 lb acre⁻¹ have been reported (2024, Bioenergy Res)—aligning sustainability goals with economic value. These attributes fit within modern genomic selection frameworks for climate-ready crops (Theor Appl Genet, 2021), where wax load and composition emerge as practical, measurable targets for breeding programs.
Cuticular waxes are integral to plant adaptation—reducing water loss, mitigating heat/UV, resisting pests, and preserving postharvest quality. Advances in GC-MS/GC×GC-MS profiling now resolve wax composition with high specificity, enabling mechanistic insights and trait deployment in breeding and storage management. As climate variability intensifies, wax traits offer a practical lever for resilient, productive crops.
We offer specialized GC-based wax profiling services to support plant biology research and crop innovation. Contact us to learn more.
11. FAQs on Cuticular Waxes
Q1: What is the main function of cuticular wax in plants?
Cuticular wax prevents water loss, reflects UV light, and provides protection against pathogens and insects.
Q2: How are plant waxes analyzed in research?
They are commonly extracted using organic solvents and analyzed using GC-MS or GC×GC-MS techniques.
Q3: Do all plants have the same wax composition?
No. Wax composition varies by species, tissue type, development stage, and environmental conditions.
Q4: Can wax traits improve crop drought tolerance?
Yes. Thicker or more structured wax layers reduce transpiration and are linked to improved drought survival.
Q5: Are cuticular waxes relevant to fruit storage?
Yes. They help preserve firmness, reduce dehydration, and prevent chilling injury during postharvest storage.
Q6: How should I report wax amounts—per area or per weight?
For leaves, µg cm⁻² is standard; for fruits, use µg cm⁻² or µg g⁻¹ FW and state the choice clearly.
Q7: Best solvent for surface-specific extraction—hexane or chloroform?
Hexane is gentler and reduces deeper extraction; chloroform extracts more but increases artifact risk. Keep dips short (10–30 s).
Q8: Which genes indicate an active alkane-forming pathway?
CER1/CER3 (with CYTB5) for alkanes; FAR/WSD1 for alcohol/ester branch; MYB94/MYB96/WIN1 as upstream regulators.
References
- Jenks MA, Ashworth EN. Plant Epicuticular Waxes: Function, Production, and Genetics. Hortic Rev (Am Soc Hortic Sci). 1999;23:1-68. https://doi.org/10.1002/9780470650752.ch1.
- Shepherd T, Wynne Griffiths D. The Effects of Stress on Plant Cuticular Waxes. New Phytol. 2006;171(3):469-499. doi:10.1111/j.1469-8137.2006.01826.x.
- Yeats TH, Rose JKC. The Formation and Function of Plant Cuticles. Plant Physiol. 2013;163(1):5-20. doi:10.1104/pp.113.222737.
- Bernard A, Joubès J. Arabidopsis Cuticular Waxes: Advances in Synthesis, Export, and Regulation. Prog Lipid Res. 2013;52(1):110-129. doi:10.1016/j.plipres.2012.10.002.
- Wang Y, Wang J, Chai G, et al. Developmental Changes in Composition and Morphology of Cuticular Waxes on Leaves and Spikes of Glossy and Glaucous Wheat (Triticum aestivum L.). PLoS One. 2015;10(10):e0141239. doi:10.1371/journal.pone.0141239.
- Trivedi P, Nguyen N, Hykkerud AL, et al. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front Plant Sci. 2019;10:431. doi:10.3389/fpls.2019.00431.
- Vráblová M, Vrábl D, Sokolová B, Marková D, Hronková M. A Modified Method for Enzymatic Isolation of and Subsequent Wax Extraction from Arabidopsis thaliana Leaf Cuticle. Plant Methods. 2020;16:129. doi:10.1186/s13007-020-00673-7.
- Scognamiglio M, Baldino L, Reverchon E. Fractional Separation and Characterization of Cuticular Waxes Extracted from Vegetable Matter Using Supercritical CO₂. Separations. 2022;9(3):80. doi:10.3390/separations9030080.
- Zheng J, He Z, Yang K, Liu Z, Zhao D, Qian MC. Volatile Analysis of Wuliangye Baijiu by LiChrolut EN SPE Fractionation Coupled with Comprehensive GC×GC-TOFMS. Molecules. 2022;27(4):1318. doi:10.3390/molecules27041318.
- Kong Q, Liu R, Wu W, Fang X, Chen H, Han Y, Chen J. Cuticular Wax Triterpenes Maintain Storage Quality of Blueberries by Reducing Water Loss. Foods. 2023;12(14):2643. doi:10.3390/foods12142643.
- Gorb EV, Gorb SN. Insect attachment on waxy plant surfaces: the effect of pad contamination by different waxes. Beilstein J Nanotechnol. 2024;15:385-395. Published 2024 Apr 11. doi:10.3762/bjnano.15.35.
- Chemelewski R, McKinley BA, Finlayson S, Mullet JE. Epicuticular wax accumulation and regulation of wax pathway gene expression during bioenergy Sorghum stem development. Front Plant Sci. 2023;14:1227859. Published 2023 Oct 23. doi:10.3389/fpls.2023.1227859.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


