Polyphenols Explained: How Plant Compounds Boost Health and Wellness
Polyphenols are a broad family of naturally occurring phytochemicals best known as the antioxidants in colorful fruits, tea, wine, and chocolate. These compounds give plants their vivid pigments and astringent taste, but they also offer remarkable health benefits to humans. Researchers are drawn to polyphenols for their potential in preventing chronic diseases – from cancer to heart disease – thanks to their anti-inflammatory and antioxidant actions. Polyphenols are ubiquitous in our diet and environment, yet their significance spans from plant survival to human wellness. In this blog, we will explore the story of polyphenols:
- Polyphenols Definition, Discovery & Classification
- Phenylpropanoid Pathway Polyphenols — Plant Biosynthesis & Regulation
- Polyphenol Bioavailability and Gut Microbiome Metabolism
- Polyphenols in Cancer Research
- Polyphenols in Neurodegeneration
- Polyphenols in Metabolic Syndrome & Type 2 Diabetes
- Polyphenols in Cardiovascular Disease
- Polyphenols in Plant
- From Superfoods to Skincare: Polyphenols in Daily Life
- Polyphenol Profiling — Detection Methods
Polyphenols Definition, Discovery & Classification
what are Polyphenols
_1757407148_WNo_201d316.webp) Polyphenols (plant phenolic compounds) are a vast superfamily of secondary metabolites characterized by multiple phenolic rings bearing hydroxyl groups. Chemically, they range from small phenolic acids to large polymers (tannins). Biosynthetically, most derive from the shikimate → phenylpropanoid pathway with contributions from the acetate–malonate (polyketide) pathway, yielding core intermediates such as cinnamic acids and p-coumaroyl-CoA that feed into flavonoids, stilbenes, lignans, and tannins. Functionally, polyphenols act as antioxidants, metal chelators, signaling modulators, and defense molecules in plants, and exhibit anti-inflammatory, cardioprotective, neuroprotective, and chemopreventive activities in humans.
Polyphenols (plant phenolic compounds) are a vast superfamily of secondary metabolites characterized by multiple phenolic rings bearing hydroxyl groups. Chemically, they range from small phenolic acids to large polymers (tannins). Biosynthetically, most derive from the shikimate → phenylpropanoid pathway with contributions from the acetate–malonate (polyketide) pathway, yielding core intermediates such as cinnamic acids and p-coumaroyl-CoA that feed into flavonoids, stilbenes, lignans, and tannins. Functionally, polyphenols act as antioxidants, metal chelators, signaling modulators, and defense molecules in plants, and exhibit anti-inflammatory, cardioprotective, neuroprotective, and chemopreventive activities in humans.
Discovery of Polyphenols
From tanning extracts to nutraceutical leads, the polyphenol story spans two centuries. In 1831, Henri Braconnot isolated gallic and ellagic acids from oak galls, anchoring tannins to defined small molecules. In the 1930s, Albert Szent-Györgyi traced capillary-protective activity in citrus/paprika to flavonoids—briefly dubbed “vitamin P”—catalyzing biomedical interest. Mid-century, Edgar Bate-Smith, Tony Swain, and Edwin Haslam unified plant “polyphenols” by shared structural criteria (formalized by Haslam in 1966), shifting the field from folk chemistry to molecular taxonomy. In 1992, the French Paradox spotlighted red-wine resveratrol and dietary polyphenols in cardiometabolic health. Since then, chromatography–mass spectrometry and metabolomics have expanded known plant polyphenols beyond 10,000 entities and linked them to biosynthetic pathways, bioavailability, and microbiome-derived metabolites—laying today’s foundation for mechanism-driven nutrition and therapeutics.
Polyphenols Classification
Polyphenols are molecules bearing multiple phenolic (aromatic + –OH) units; this shared chemistry underlies redox behavior, metal chelation, and protein interactions. The field typically groups them into five major subclasses:
Flavonoids (C₆–C₃–C₆; A/B aromatic rings + heterocyclic C-ring): Largest class. Subtypes include flavonols (quercetin), flavones (apigenin), flavanones (naringenin), isoflavones (daidzein), flavan-3-ols (catechins/procyanidins), anthocyanidins (pigments). Features: red/blue/yellow pigments, ROS scavenging, enzyme/receptor modulation, strong structure–activity tuning via hydroxylation/glycosylation/acylation.
Phenolic acids (single ring):
• Hydroxybenzoic acids (C₆–C₁)—e.g., gallic acid, ellagic acid; often in berries/tea; precursors to hydrolyzable tannins.
• Hydroxycinnamic acids (C₆–C₃)—e.g., caffeic, ferulic, p-coumaric, chlorogenic acids; abundant in coffee, cereals; antioxidant and cell-wall–linked derivatives.
Stilbenes (C₆–C₂–C₆ with ethylene bridge): Prototype resveratrol; typically phytoalexins induced by stress; conjugation (glycosides, methylation) governs stability and bioavailability.
Lignans (dimerized C₆–C₃–C₆–C₃ frameworks): Formed by phenylpropanoid radical coupling; dietary precursors (e.g., secoisolariciresinol) converted by gut microbiota to enterolignans with estrogenic and antioxidant properties.
Tannins (high-MW polyphenols, protein-binding):
• Condensed tannins (proanthocyanidins): polymers of flavan-3-ols (catechin/epicatechin).
• Hydrolyzable tannins: gallotannins/ellagitannins (gallic/hexahydroxydiphenic acid esters on sugars). Features: astringency, protein precipitation, strong antioxidant/antimicrobial activity.
Phenylpropanoid Pathway Polyphenols — Plant Biosynthesis & Regulation
In plants, most polyphenols arise from the phenylpropanoid pathway, which channels carbon from primary metabolism (PEP + E4P → shikimate → aromatic amino acids) into specialized phenolics. The gateway step is PAL converting L-phenylalanine to trans-cinnamic acid, followed by C4H and 4CL to p-coumaroyl-CoA, a branchpoint that feeds flavonoids, stilbenes, lignans, coumarins and precursors of tannins. Flux into flavonoids is amplified by the polyketide (acetate–malonate) route, where CHS condenses p-coumaroyl-CoA with malonyl-CoA; STS competes toward stilbenes. Regulation is multilayered: MYB–bHLH–WD40 transcriptional modules, enzyme gene families (PAL/C4H/4CL/CHS/CHI/F3H/FLS/DFR/ANS, etc.), light/UV, temperature, heavy metals, and phytohormones collectively tune pathway output and localization (e.g., vacuolar sequestration via transporters). This architecture links environment and development to polyphenol quantity and composition across tissues and species.
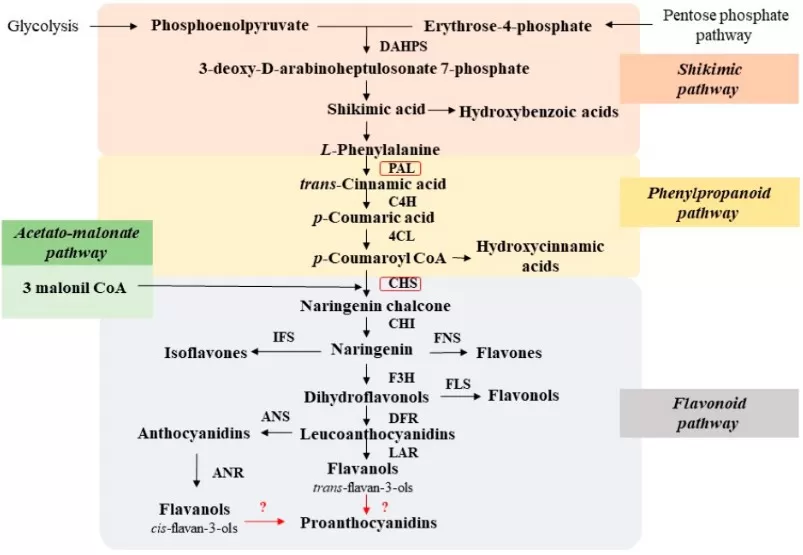
Biosynthesis Pathway of Phenolic
Source: Zagoskina NV, Zubova MY, Nechaeva TL, Kazantseva VV, Goncharuk EA, Katanskaya VM, Baranova EN, Aksenova MA. “Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications,” International Journal of Molecular Sciences (2023) 24(18):13874, Figure 8. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Polyphenol Bioavailability and Gut Microbiome Metabolism
After ingestion, most dietary polyphenols arrive as glycosides or polymers with limited small-intestinal absorption. Aglycones released by brush-border or microbial enzymes enter enterocytes and undergo rapid Phase II conjugation—primarily glucuronidation (UGTs), sulfation (SULTs), and O-methylation (COMT)—and further conjugation in the liver, so circulating species are largely glucuronide/sulfate/methyl metabolites rather than parent compounds. Conjugates can be secreted into bile and recirculated (enterohepatic cycling). Unabsorbed polyphenols reach the colon, where the gut microbiota perform deglycosylation, ring-cleavage, and dehydroxylation to smaller phenolic acids or urolithins that may be reabsorbed; inter-individual “metabotypes” drive variability in exposure and effects. In tissues, sulfatases/glucuronidases may locally regenerate aglycones from conjugates. Elimination occurs mainly via urine (phase-II conjugates and microbial phenolics) and feces (unabsorbed residues and biliary returns). This host–microbiome co-metabolism explains the modest bioavailability of parent polyphenols yet diverse systemic activities of their metabolites.
_1757407390_WNo_1173d860.webp)
Human Polyphenol Metabolism: Absorption, Phase II Conjugation, Microbial Biotransformation, and Enterohepatic Cycling (schematic)
Source: Scott MB, Styring AK, McCullagh JSO. “Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals,” Pathogens (2022) 11(7):770, Figure 1. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Across human health, dietary polyphenols act as multi-target modulators: tuning redox and inflammatory signaling, epigenetics, mitochondria, and the gut microbiome. Evidence spans cancer chemoprevention/adjuvant therapy, neuroprotection, metabolic syndrome, and cardiovascular atheroprotection—where bioavailability and microbiome-derived metabolites (e.g., urolithin A) shape outcomes.
Polyphenols in Cancer Research (Multi-target Chemoprevention & Adjuvant Therapy)
Dietary polyphenols (e.g., EGCG, curcumin, resveratrol, quercetin) act as multi-target modulators across cancer hallmarks. They trigger apoptosis and cell-cycle arrest; suppress proliferative signaling (PI3K/Akt/mTOR, EGFR/ERK); inhibit NF-κB–driven inflammation, angiogenesis (↓VEGF), and metastasis (↓MMPs); and reprogram tumor epigenetics by inhibiting DNMTs/HDACs. Beyond tumor-intrinsic effects, polyphenols can sensitize tumors to chemotherapy and radiotherapy and modulate antitumor immunity (macrophages, T/NK cells). Their low toxicity profiles and pathway breadth make them attractive for chemoprevention and as adjuncts to standard of care, though dose, formulation, and bioavailability remain key translational variables.
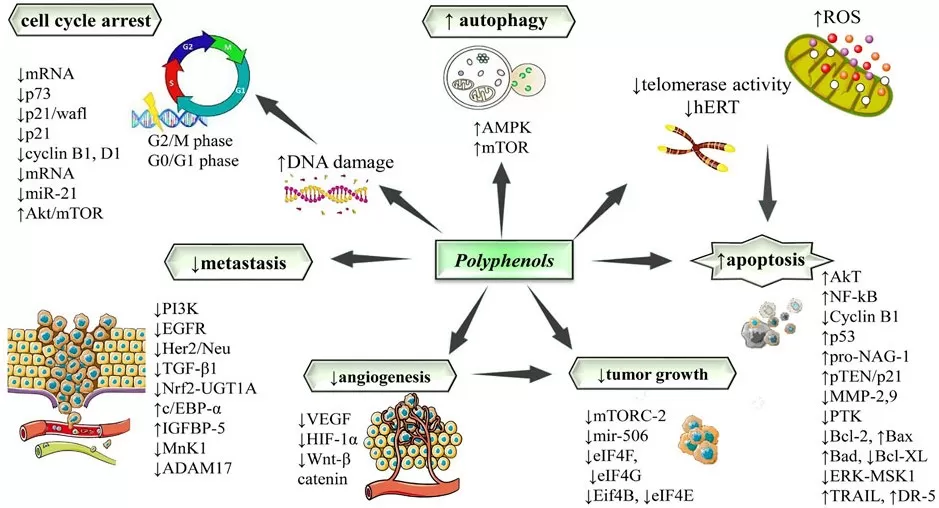
Anticancer mechanisms of polyphenols
Source: Sharma E, Attri DC, Sati P, et al. “Recent updates on anticancer mechanisms of polyphenols,” Frontiers in Cell and Developmental Biology (2022) 10:1005910, Figure 2. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
In head-and-neck squamous cell carcinoma (FaDu xenograft), dietary EGCG markedly suppressed tumor growth: mice showed smaller tumor volumes and weights without body-weight loss. Mechanistically, EGCG reduced global DNA methylation (↓5-mC; ↑5-hmC) and decreased DNMT1/3A/3B, with reactivation of tumor-suppressor proteins (p16^INK4a^, p21^Waf1/Cip1^, p27^Kip1^). These in vivo data support an epigenetic mechanism for polyphenol anticancer action alongside growth inhibition, aligning with its chemosensitizing potential reported elsewhere.
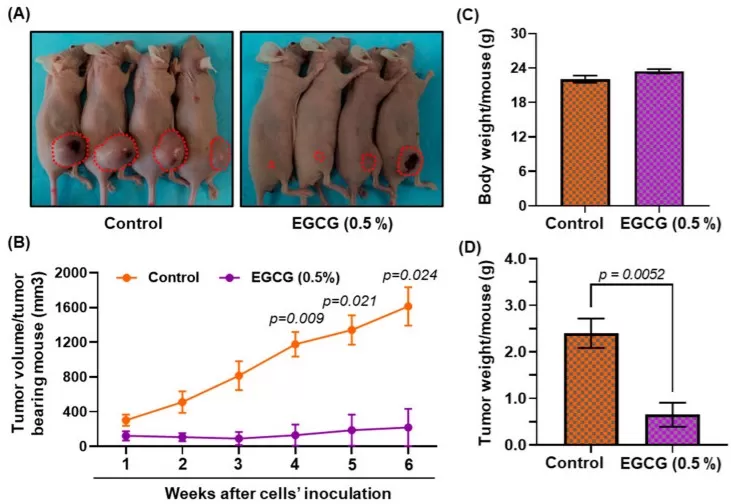
Dietary EGCG suppresses FaDu xenograft growth and reduces tumor burden
Source: Agarwal A, Kansal V, Farooqi H, Prasad R, Singh VK. “Epigallocatechin Gallate (EGCG), an Active Phenolic Compound of Green Tea, Inhibits Tumor Growth of Head and Neck Cancer Cells by Targeting DNA Hypermethylation,” Biomedicines (2023) 11(3):789, Figure 6. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Polyphenols in Neurodegeneration (Oxidative, Proteostatic & Neuroimmune Control)
In Alzheimer’s and Parkinson’s models, polyphenols mitigate oxidative stress (direct ROS/RNS scavenging, metal chelation) and activate cytoprotective programs (Nrf2/ARE, mitochondrial biogenesis). They modulate microglia and astrocytes to dampen NF-κB/MAPK signaling, lowering neurotoxic cytokines. Several molecules interfere with proteotoxic cascades: inhibiting Aβ oligomerization/fibrillization, reducing tau hyperphosphorylation, and promoting autophagic clearance via SIRT1–AMPK. Cerebrovascular benefits (↑eNOS/NO, improved neurovascular coupling) and enhanced neurotrophic support (e.g., BDNF) further sustain synaptic function. Although bioavailability of parent aglycones is limited, circulating conjugates and microbiome-derived metabolites (urolithins, phenylacids) reach the CNS or act peripherally to reduce neuroinflammation. Collectively, polyphenols offer a multi-pronged strategy to slow brain aging and complement disease-modifying therapies.
Polyphenols in Metabolic Syndrome & Type 2 Diabetes (Insulin Signaling & Gut–Liver Axis)
Polyphenols improve insulin sensitivity by activating AMPK and downstream pathways that enhance GLUT4 translocation, suppress hepatic gluconeogenesis, and shift lipid metabolism toward oxidation (↓SREBP1c/ACC; ↑PPARα). In adipose and liver, they quell low-grade inflammation by inhibiting NF-κB and NLRP3 signaling, lowering TNF-α/IL-6 and oxidative stress. Polyphenols reshape the gut microbiome (↑Bifidobacterium/Akkermansia; ↓endotoxemia), strengthen barrier integrity, and yield bioactive metabolites (e.g., urolithin A, phenylpropionates) that signal through GPCRs and nuclear receptors to improve glucose–lipid homeostasis. Clinically, diets rich in flavonoids, anthocyanins, and procyanidins associate with modest reductions in fasting glucose, HbA1c, hepatic steatosis, and blood pressure. Formulation strategies (glycosides, nanoemulsions) and timing with meals can enhance exposure and cardiometabolic impact.
Polyphenols in Cardiovascular Disease (Endothelial Function & Atheroprotection)
Cardiovascular protection arises from antioxidant and anti-inflammatory actions that reduce LDL oxidation, foam-cell formation, and vascular ROS. Polyphenols improve endothelial function by activating eNOS (via AKT/AMPK phosphorylation), increasing NO bioavailability, and restoring flow-mediated dilation, which translates to lower blood pressure. They downregulate adhesion molecules (VCAM-1/ICAM-1) and chemokines, stabilize plaques by shifting macrophages toward M2 phenotypes, and inhibit MMPs that drive instability. Lipid handling improves through reduced intestinal absorption, enhanced hepatic LDL receptor expression, and better HDL efflux capacity. Some polyphenols also attenuate platelet activation and aggregation, lowering thrombotic risk. Together, these mechanisms explain epidemiologic links between high-polyphenol dietary patterns (e.g., Mediterranean diet; tea/cocoa intake) and reduced incidence of coronary events and stroke.
Polyphenols in Plant
Plant polyphenols are central to survival strategies and ecological fitness. As defense molecules, phenylpropanoid derivatives (flavonoids, stilbenes, lignans, tannins) deter pathogens and herbivores, reinforce barriers, and act as phytoalexins. Their photoprotective roles are equally vital: epidermal flavonols and anthocyanins screen UV and buffer ROS surges, with UV-B rapidly inducing both pathway genes and end products. In blueberry calli, 24 h of UV-B elevates flavonols, proanthocyanidins, and anthocyanins several-fold, illustrating stress-responsive carbon flux into phenylpropanoids. Regulation is multilayered: MBW (R2R3-MYB–bHLH–WD40) transcriptional modules coordinate structural genes (PAL, C4H, 4CL, CHS, CHI, F3H/FLS/UFGT, etc.), while hormones and light signals tune kinetics and tissue specificity. Methodologically, polyphenols are tractable readouts for metabolomics × transcriptomics, enabling discovery of key regulators, stress markers, and breeding targets for nutrition and resilience. Collectively, this network links environment and development to quantitative, class-specific polyphenol profiles across organs and species.
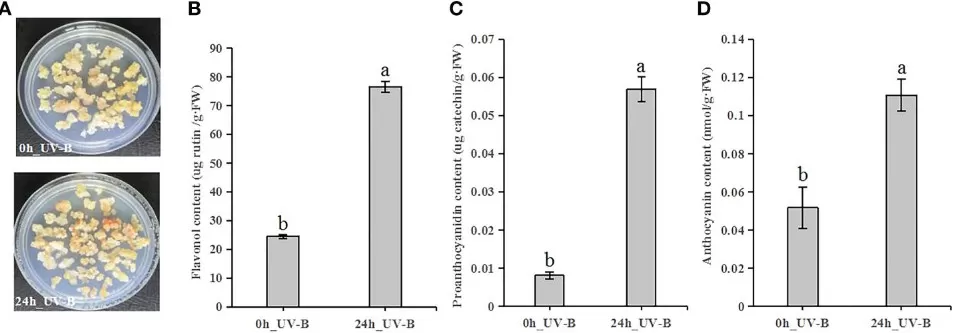
UV-B exposure induces flavonoid accumulation in blueberry calli.
Source: Source: Luo X, Tian H, Hu X, et al. “UV-B induces the expression of flavonoid biosynthetic pathways in blueberry (Vaccinium corymbosum) calli,” Frontiers in Plant Science (2022) 13:1079087, Figure 3. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
From Superfoods to Skincare: Polyphenols in Daily Life
Polyphenols are the bioactive backbone of many functional foods. Diets rich in berries, grapes, apples, citrus, leafy greens, tea/coffee, cocoa, nuts, and olive oil deliver diverse phenolic profiles linked to cardiometabolic and cognitive benefits. Beyond whole foods, nutraceuticals (e.g., quercetin, curcumin, grape seed procyanidins, EGCG) are formulated as capsules or beverages; for researchers and product developers, efficacy hinges on bioavailability—hence the rise of phytosomes, nanoemulsions, and co-crystals to improve absorption and tissue delivery.
In cosmetics, polyphenols function as antioxidative and photoprotective actives that limit UV-induced ROS, preserve collagen (anti-collagenase/elastase), and calm inflammation. Common actives include green-tea catechins, resveratrol, and coffeeberry extracts, used in sunscreens, serums, and barrier-care products.
Other applications span food technology and materials: rosemary/tea extracts as natural preservatives and color stabilizers; wine and cocoa processing optimized around tannin/anthocyanin profiles for mouthfeel, color, and aging; exploratory uses in biobased coatings and drug-delivery carriers leveraging phenolic redox chemistry and metal chelation.
Polyphenol Profiling — Detection Methods
Targeted LC-MS/MS (UHPLC-QqQ, MRM). The gold standard for targeted polyphenol quantification across flavonoids, phenolic acids, stilbenes, lignans, and key microbial metabolites (e.g., urolithin A, phenyl-γ-valerolactones). Works for foods, plant tissues, plasma/serum, urine, and feces. High specificity/sensitivity and wide dynamic range; requires authentic standards and isotope-labeled IS to control matrix effects.
Untargeted HRMS (Orbitrap/TOF). Discovery-first profiling of phenolic compounds; detects thousands of features and annotates via MS/MS libraries. Ideal for variety comparisons or intervention studies; semi-quantitative and needs careful ID confidence workflows. Often guides the design of LC-MS/MS polyphenol profiling panels.
HPLC-DAD (UV/Vis). Rapid class profiling (e.g., 280 nm “total phenolics,” 520 nm anthocyanins). Useful for QC trending and quick screens; lower specificity and potential co-elution vs MS.
GC-MS (derivatized). Best for volatile/low-MW phenolics or phenolic acids after silylation. Excellent separation; requires derivatization and is unsuitable for polymers.
Folin–Ciocalteu & antioxidant assays (DPPH/ORAC/ABTS). Fast, inexpensive screening of “total phenolics”/antioxidant capacity; non-specific and cannot substitute for LC-MS/MS when linking intake to exposure.
Sample prep & QC. Acidified 70–80% MeOH/MeCN extraction; optional enzymatic/acid hydrolysis vs direct conjugate measurement; SPE cleanup; reversed-phase LC with negative-mode ESI; retention-time scheduling; stable-isotope IS; external calibration (linearity), LOD/LOQ, recovery, matrix-effect, carryover, and stability checks. Report foods in mg/100 g FW; biofluids in ng/mL–μg/mL with full QC deliverables.
Targeted LC-MS/MS (UHPLC-QqQ, MRM) quantifies panels across flavonoids (quercetin/kaempferol/catechins/EGCG), phenolic acids (gallic/caffeic/ferulic), stilbenes (resveratrol), lignans, and microbial metabolites (urolithin A, phenyl-γ-valerolactones). Matrices include foods, plant tissues, plasma/serum, urine, and feces. Typical prep: acidified MeOH/MeCN extraction, optional enzymatic or acid hydrolysis vs direct conjugate quantification, SPE cleanup, negative-mode ESI, isotope-labeled IS, linear calibration with LOD/LOQ and matrix-effect checks. Untargeted HRMS (Orbitrap/TOF) supports discovery of untargeted metabolomics phenolic compounds and guides targeted panel design. Classical Folin–Ciocalteu is non-specific; use for screening, not intake inference. Report mg/100 g FW (foods) and ng/mL or μg/mL (biofluids) with full QC deliverables.
Metabolomics Spotlight: Unleashing Polyphenols with MetwareBio
Polyphenols underpin advances across human health (cardio-metabolic, neuro, oncology), plant science (defense, stress tolerance, pigmentation), and food/nutraceuticals (quality, authenticity, efficacy). To turn these insights into data, MetwareBio the proteomics and metabolomics company, offers widely-targeted metabolomics for plants that profiles polyphenols with high coverage and rigor. Our plant-specific in-house database (MWDB) now indexes >61,000 plant metabolites, enabling confident IDs at scale. Using high-resolution MS/MS for spectral matching and QQQ-MRM for precise quantification, we deliver accurate identification and robust numbers—typically thousands of metabolites per sample—under a mature QC system.
Polyphenols FAQs
Q1. What are polyphenols and their benefits?
Polyphenols are plant molecules with multiple phenolic units; polyphenols benefits include vascular support, anti-inflammatory signaling, and microbiome-mediated effects.
Q2. Which foods are highest in polyphenols (polyphenols food list)?
By intake: tea polyphenols and coffee polyphenols, plus berries, apples, nuts; per-100 g leaders include spices and cocoa powders.
Q3. What are high polyphenol foods for everyday diets?
Berries, grapes, citrus, leafy greens, dark chocolate polyphenols, nuts, extra-virgin olive oil, tea, and coffee—rotate for diversity.
Q4. Is olive oil high in polyphenols (high-polyphenol olive oil)?
Yes—look for EVOO delivering ≥5 mg hydroxytyrosol/20 g; early-harvest, robust oils more often qualify.
Q5. Do cocoa flavanols lower blood pressure (cocoa flavanols benefits)?
Regular intake around ~200 mg/day cocoa flavanols improves endothelial function and can modestly reduce BP.
Q6. Tea vs coffee vs red wine: which has more polyphenols?
A cup of coffee or tea typically supplies ~100–300 mg; red wine offers less and includes alcohol—use grapes/berries for similar phenolics.
Q7. What is the bioavailability of polyphenols?
Parent aglycones are limited; bioavailability of polyphenols mainly comes from conjugates and gut-microbiome metabolites reaching circulation.
Q8. How do polyphenols interact with the gut microbiome (polyphenols and gut microbiome)?
They act as substrates/modulators, fostering beneficial taxa and yielding urolithin A and phenyl-acids that signal systemically.
Q9. Food vs supplements: which is better for polyphenols benefits?
Choose food-first for matrix synergy; supplements suit targeted dosing or research endpoints.
Q10. How much polyphenols per day is optimal?
Plant-rich diets typically deliver hundreds of mg to ~1 g/day; no official RDA exists—focus on diverse sources.
Q11. Does cooking reduce polyphenols (tea/coffee brewing, vegetables)?
Often—boiling leaches/degrades; steaming/microwaving preserves more; brew tea 3–5 min to extract catechins.
Q12. What are common polyphenol compounds (resveratrol, quercetin, ellagitannins)?
Key examples: resveratrol (stilbene), quercetin (flavonol), ellagitannins (tannins → urolithin A via microbiome), and cocoa flavanols.
References
- Zagoskina NV, Zubova MY, Nechaeva TL, et al. Polyphenols in plants: structure, biosynthesis, abiotic stress regulation, and practical applications. Int J Mol Sci. 2023;24(18):13874. doi:10.3390/ijms241813874
- Scott MB, Styring AK, McCullagh JSO. Polyphenols: bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens. 2022;11(7):770. doi:10.3390/pathogens11070770
- Sharma E, Attri DC, Sati P, et al. Recent updates on anticancer mechanisms of polyphenols. Front Cell Dev Biol. 2022;10:1005910. doi:10.3389/fcell.2022.1005910
- Agarwal A, Kansal V, Farooqi H, Prasad R, Singh VK. Epigallocatechin Gallate (EGCG), an active phenolic compound of green tea, inhibits tumor growth of head and neck cancer cells by targeting DNA hypermethylation. Biomedicines. 2023;11(3):789. doi:10.3390/biomedicines11030789
- Luo X, Tian H, Hu X, et al. UV-B induces the expression of flavonoid biosynthetic pathways in blueberry (Vaccinium corymbosum) calli. Front Plant Sci. 2022;13:1079087. doi:10.3389/fpls.2022.1079087
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


