Proteomics and Metabolomics/Lipidomics in Metabolic Disease Research: Insights and Applications
Metabolic diseases—including type 2 diabetes (T2D), metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD/MASLD)—represent a severe global health burden, with T2D complications alone contributing significantly to rising cardiovascular mortality. These disorders are characterized by molecular hallmarks such as impaired insulin signaling, chronic low-grade inflammation, lipid imbalance, mitochondrial dysfunction, and oxidative stress. Traditional single-layer approaches fail to capture the multifactorial and network-level nature of these pathologies. Multi-omics, particularly proteomics and metabolomics, has therefore become indispensable for understanding disease mechanisms. Proteomics provides a systematic view of protein abundance, isoforms, and post-translational modifications, uncovering altered signaling cascades, while metabolomics captures downstream metabolic outputs that reflect real-time flux through glucose, lipid, and amino acid pathways [3,8]. Together, they enable early biomarker discovery, stratification of heterogeneous patient populations, and the identification of therapeutic targets.
Proteomics in Metabolic Disease
Proteomics provides critical insights into protein-level disruptions that drive metabolic pathologies, including dysregulated insulin receptor substrates, pro-inflammatory cytokines, and lipid-processing enzymes. Discovery proteomics, enabled by high-resolution LC-MS/MS and data-independent acquisition (DIA), offers an unbiased and comprehensive survey of the proteome, allowing the identification of novel proteins linked to disease onset and progression. In contrast, targeted proteomics—such as parallel reaction monitoring (PRM)—delivers high sensitivity and reproducibility for quantifying predefined protein panels, ensuring robust validation of candidate biomarkers. These complementary approaches are particularly valuable in metabolic diseases, where complex protein networks involving signaling kinases, transporters, and mitochondrial enzymes underlie distinct phenotypes. In T2D, large-scale proteomic and phosphoproteomic profiling of skeletal muscle (>120 individuals) revealed fasting protein signatures predictive of insulin sensitivity and highlighted preserved versus dysregulated signaling nodes in insulin-resistant states [1]. In NAFLD, plasma proteomics distinguishes between simple steatosis (NAFL) and cirrhosis, while proteins such as FGF-21 show strong associations with fibrosis and steatosis severity [2]. More recently, advanced methods such as SomaScan® aptamer-based assays have enabled high-throughput “liquid biopsy” analyses of thousands of serum proteins simultaneously, supporting noninvasive diagnostics for NAFLD histological features and longitudinal monitoring of therapeutic responses [3].
Metabolomics and Lipidomics in Metabolic Disease
Metabolomics and lipidomics captures dynamic disturbances in metabolic pathways that define disease progression, including impaired glucose homeostasis, altered lipid oxidation, disrupted bile acid turnover, and mitochondrial energy dysfunction. In NAFLD and MASH, consistent findings include dysregulated amino acid metabolism and profound lipid remodeling—manifested as elevated fatty acids, triglycerides, phospholipids, and bile acids—reflecting hepatic overload and mitochondrial impairment. Specific metabolites, such as sphingosine [So(d18:1)], are elevated in NASH and contribute to liver fibrosis [4], while intermediates like β-hydroxyisobutyric acid and 3-methyl-2-oxovaleric acid function as “metabokines,” linking adipose tissue browning to systemic metabolic regulation [5]. Beyond these disease-associated signatures, methodological approaches in metabolomics differ in scope and precision. Untargeted metabolomics, leveraging high-resolution LC-MS, enables global, unbiased mapping of the metabolome, facilitating hypothesis generation and novel biomarker discovery. By contrast, targeted metabolomics focuses on predefined metabolites—such as amino acids, acylcarnitines, and bile acids—providing quantitative accuracy, high reproducibility, and strong translational potential. Increasingly, hybrid strategies that integrate untargeted discovery with targeted validation have proven especially powerful in metabolic disease research. For instance, in MASLD, untargeted plasma metabolomics identified cholesterol derivatives like βCDCA as key mediators linked to Akkermansia muciniphila depletion [6], while subsequent targeted validation enabled diagnostic scoring models that combined clinical parameters with hundreds of metabolites to predict MASH severity and liver-related mortality [7,8].
Why Multi-Omics in Metabolic Disease?
Integrating proteomics and metabolomics provides complementary insights by linking protein-level regulation with downstream metabolic flux, yielding causal interpretations of disease mechanisms. Proteomics highlights upstream regulators such as kinases and signaling proteins, while metabolomics reflects functional consequences in terms of pathway activity and metabolic imbalance. This synergy has advanced biomarker research, as multi-omics scores combining proteomic and metabolomic data outperform single-omics approaches in predicting disease progression and severity [8]. Furthermore, integration supports precision stratification: proteomic signatures can differentiate T2D subtypes with distinct insulin resistance mechanisms [1], while metabolomic profiles identify subgroups predisposed to NAFLD progression [9,10]. Systems-level integration also dissects complex host–microbiome–metabolome interactions, as demonstrated by multi-omics analyses linking gut microbial metabolites to obesity-associated metabolic dysregulation [11-13]. Although challenges remain in large-scale data integration, biomarker validation, and clinical implementation, multi-omics clearly enhances our capacity to model the complexity of metabolic disease and develop precision diagnostic and therapeutic strategies.
Applications of Proteomics, Metabolomics, and Lipidomics in Metabolic Disorders
The combined use of proteomics, metabolomics, and lipidomics has become indispensable for dissecting the molecular complexity of metabolic disorders. These complementary approaches not only reveal protein networks, metabolite fluxes, and lipid remodeling in isolation, but also illuminate how these layers interact to drive disease processes. Their application has yielded crucial insights across multiple disease areas, including impaired glucose metabolism and type 2 diabetes, lipid accumulation in NAFLD and NASH, disrupted energy balance in obesity, mitochondrial dysfunction affecting cellular fuel supply, and the systemic disturbances that underpin metabolic syndrome and cardiovascular disease. By capturing this breadth of information, omics technologies provide a powerful framework to uncover mechanisms, identify biomarkers, and inform therapeutic strategies in metabolic research.
Glucose Metabolism & Type 2 Diabetes
Type 2 diabetes (T2D) is a growing global health challenge, particularly in China, where prevalence continues to rise and effective prediction and prevention strategies are urgently needed. In 2024, Diabetes Care published the article “Proteomic analyses in diverse populations improved risk prediction and identified new drug targets for type 2 diabetes” by Yao et al., which investigated novel protein biomarkers for T2D [14]. The study analyzed baseline plasma from 2,026 participants of the China Kadoorie Biobank, identifying 92 incident T2D cases during follow-up. Using OLINK Explore proteomics, levels of 2,923 proteins were measured, and 33 proteins were found to be significantly associated with diabetes risk. Incorporating these proteins into conventional risk models markedly improved prediction accuracy (AUC increased from 0.77 to 0.88). Genetic analyses further confirmed causal relevance for ENTR1, LPL, and PON3, with LPL also supported as a potential therapeutic target. In this study, proteomics played a pivotal role by enabling biomarker discovery, enhancing risk stratification, and linking protein pathways to disease mechanisms. This work demonstrates how large-scale proteomic profiling can advance both early prediction and therapeutic innovation in metabolic disease research.
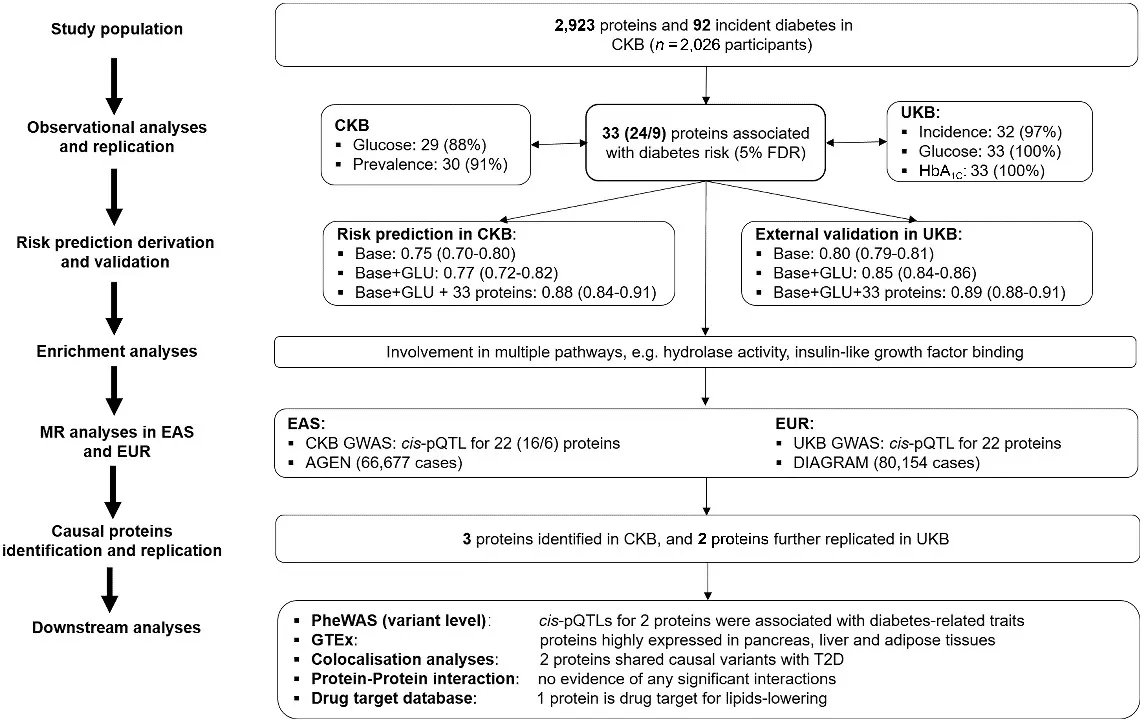
Overview of study design, analytic approaches and key findings [14]
Non-Alcoholic Fatty Liver Disease (NAFLD) & NASH
Nonalcoholic steatohepatitis (NASH), a progressive form of nonalcoholic fatty liver disease, poses a major health threat due to its links with cirrhosis and hepatocellular carcinoma, yet the mechanisms driving its progression remain poorly defined. In 2024, Hepatology published the article “Membrane phospholipid remodeling modulates nonalcoholic steatohepatitis progression by regulating mitochondrial homeostasis” by Tian et al., which revealed a key role of phospholipid metabolism in NASH pathogenesis [15]. The researchers analyzed human liver biopsies from NAFLD/NASH patients and conducted extensive experiments in liver-specific Lpcat3 knockout mice, alongside RNA sequencing, metabolomics, and lipidomics profiling. They found that LPCAT3, a phospholipid remodeling enzyme, was significantly downregulated in NASH, correlating with disease severity. Loss of Lpcat3 in mice promoted both spontaneous and diet-induced NASH and liver cancer, mediated by altered mitochondrial membrane composition, oxidative stress, and impaired homeostasis. Conversely, hepatic overexpression of Lpcat3 alleviated inflammation and fibrosis. In this study, lipidomics was central to uncovering how changes in phosphatidylcholine and cardiolipin composition disrupt mitochondrial function, directly linking membrane remodeling to disease progression. This case highlights how mass spectrometry–based multiomics can provide mechanistic insights and identify LPCAT3 as a potential therapeutic target in NASH.
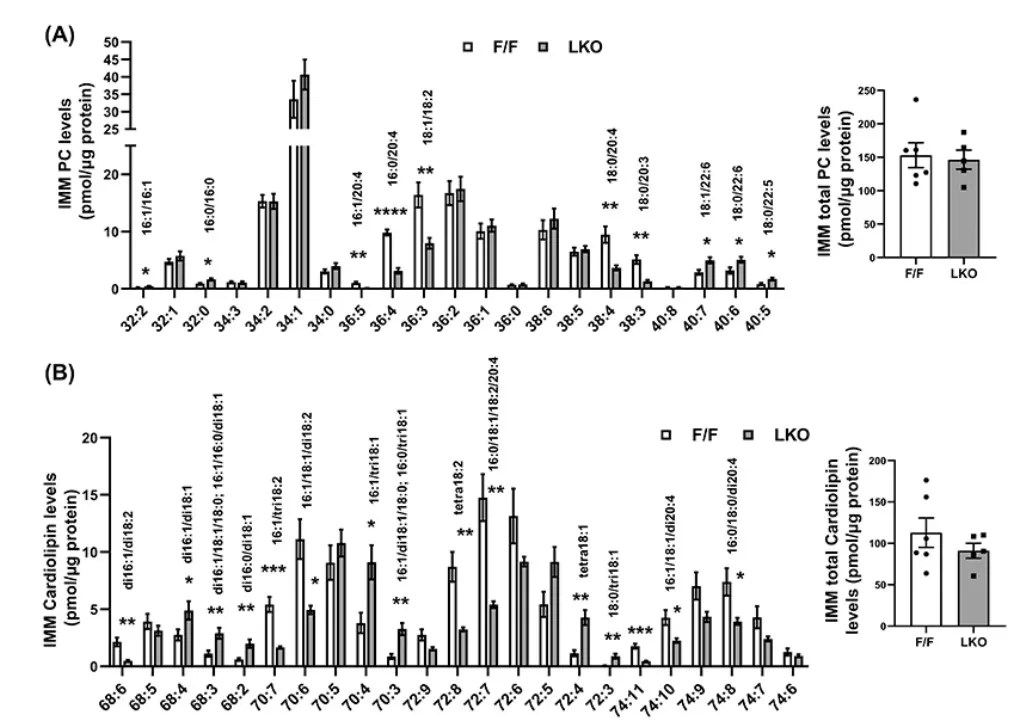
Altered Mitochondrial Phospholipid Composition in Lpcat3-Deficient NASH Mice [15]
Obesity & Lipid Metabolism Disorders
Obesity is a global health burden closely linked to insulin resistance, fatty liver disease, and type 2 diabetes, yet its molecular underpinnings remain incompletely understood. In 2023, Science Advances published the article “TNIK is a conserved regulator of glucose and lipid metabolism in obesity” by Pham et al., which identified Traf2- and NCK-interacting kinase (TNIK) as a critical regulator of energy balance [16]. The study integrated models from Drosophila and Tnik knockout mice, along with targeted metabolomics and metabolic assays, to dissect TNIK’s role in glucose and lipid handling. In flies, loss of the TNIK ortholog altered metabolite profiles and impaired de novo lipogenesis under high-sugar feeding. In mice, global Tnik deficiency conferred resistance to diet-induced obesity, insulin resistance, and hepatic steatosis, accompanied by enhanced glucose uptake in skeletal muscle and adipose tissue. Moreover, human genetic analyses revealed TNIK variants significantly associated with BMI, body fat percentage, blood glucose, and feeding behavior. In this work, metabolomics provided key mechanistic insights, uncovering widespread alterations in glycolytic, lipid, and amino acid pathways that linked TNIK to systemic metabolic control. This case demonstrates how mass spectrometry–based metabolomics can bridge genetics, physiology, and therapeutic discovery in obesity research.
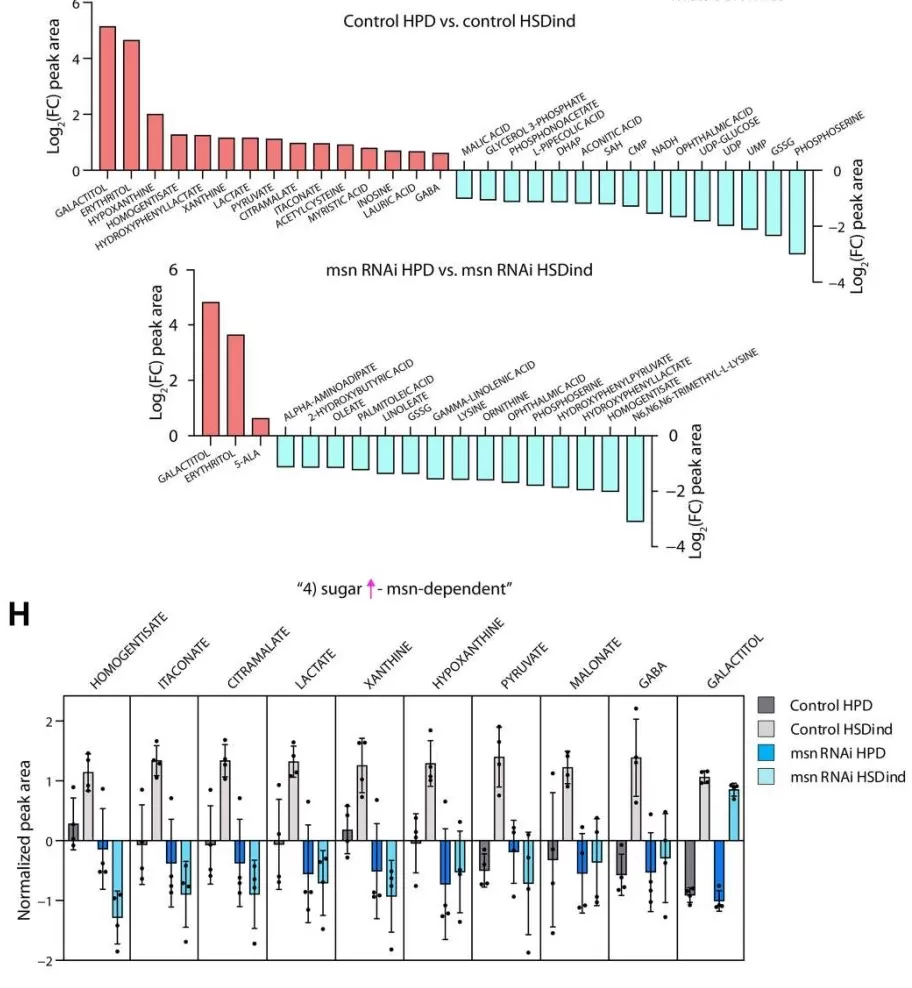
msn-Dependent Metabolomic Reprogramming Under High-Sugar Diet [16]
Mitochondrial Dysfunction & Energy Metabolism
Mitochondrial dysfunction and disrupted fatty-acid trafficking are hallmarks of metabolic diseases such as NAFLD and type 2 diabetes. In 2025, Nature Communications published Proximity proteomics reveals a mechanism of fatty acid transfer at lipid droplet–mitochondria–endoplasmic reticulum contact sites, which uncovered an ESYT1/ESYT2–VAPB complex that mediates fatty-acid transfer at LD–mitochondria–ER junctions to sustain β-oxidation [17]. Using HepG2 cells, proximity-dependent biotinylation (BioID and split-BioID) identified 71 interface proteins and, together with high-resolution imaging, localized the ESYT1/2–VAPB module to the contact sites. Functional assays in HeLa CRISPR knockouts showed that loss of ESYT1, ESYT2 or VAPB reduced LD-derived fatty-acid oxidation by 28–47%, while the double knockout caused a 67% decrease, accompanied by enlarged droplets and lipidomic evidence of acylglycerol accumulation. In vivo, precision-cut liver slices from hepatocyte-specific Esyt1 or Esyt2 knockout mice (Control n=8, Esyt1KO n=5, Esyt2KO n=7) further demonstrated impaired fatty-acid oxidation. Crucially, the combination of proteomics, lipidomics, and metabolomics allowed the researchers to link molecular machinery with metabolic flux, showing how disruption at contact sites reverberates through lipid remodeling and TCA cycle activity. This integrated omics perspective not only clarifies how mitochondria secure their fuel supply but also underscores the value of systems-level approaches for uncovering therapeutic targets in metabolic disease.
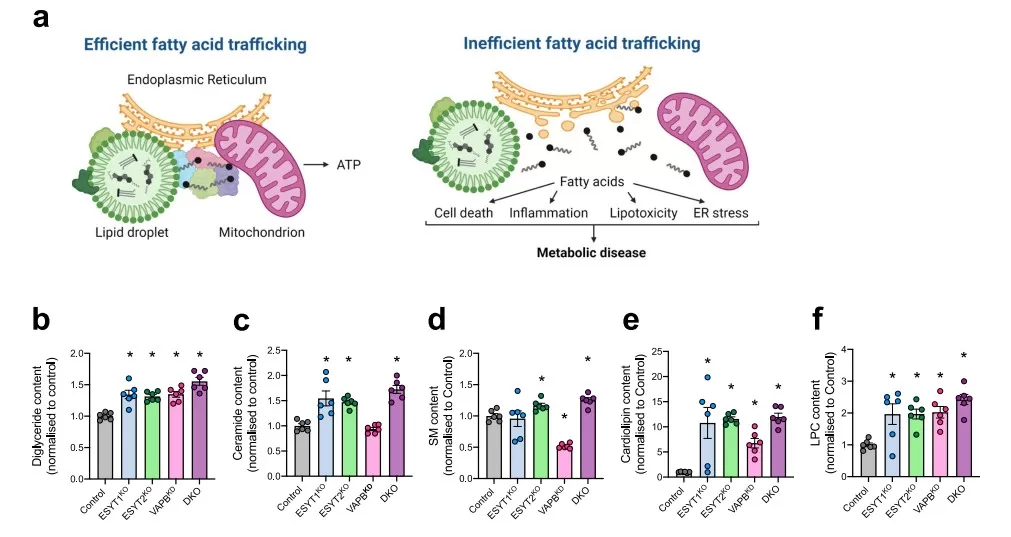
Disrupted ESYT/VAPB-Mediated Fatty Acid Trafficking Leads to Lipotoxic Lipid Accumulation [17]
Metabolic Syndrome & Cardiovascular Disease
Chronic kidney disease (CKD), a major complication of metabolic disorders such as diabetes and hypertension, markedly increases the risk of cardiovascular events and is considered a critical driver of cardiometabolic complications. In 2023, the European Heart Journal published the article “Proteomic cardiovascular risk assessment in chronic kidney disease” by Deo et al., which addressed the challenge of improving cardiovascular risk prediction in CKD patients [18]. The study analyzed 2,182 CKD participants from the Chronic Renal Insufficiency Cohort and validated findings in 485 CKD participants from the Atherosclerosis Risk in Communities study, all free of cardiovascular disease at baseline. Plasma proteomic profiling with the SomaScan v4.0 platform measured 4,638 unique proteins, and elastic net regression generated a 32-protein risk model that outperformed traditional clinical scores, achieving AUC values of 0.84–0.89 versus 0.70–0.73 over 10 years. Mendelian randomization identified 18 proteins with potential causal roles, and pathway analysis highlighted immune regulation, vascular remodeling, and hepatic fibrosis as key mechanisms. In this case, proteomics was central to discovering novel biomarkers and building a clinically superior risk model, underscoring its value for understanding mechanisms and enhancing risk stratification in cardiovascular complications of metabolic disease.
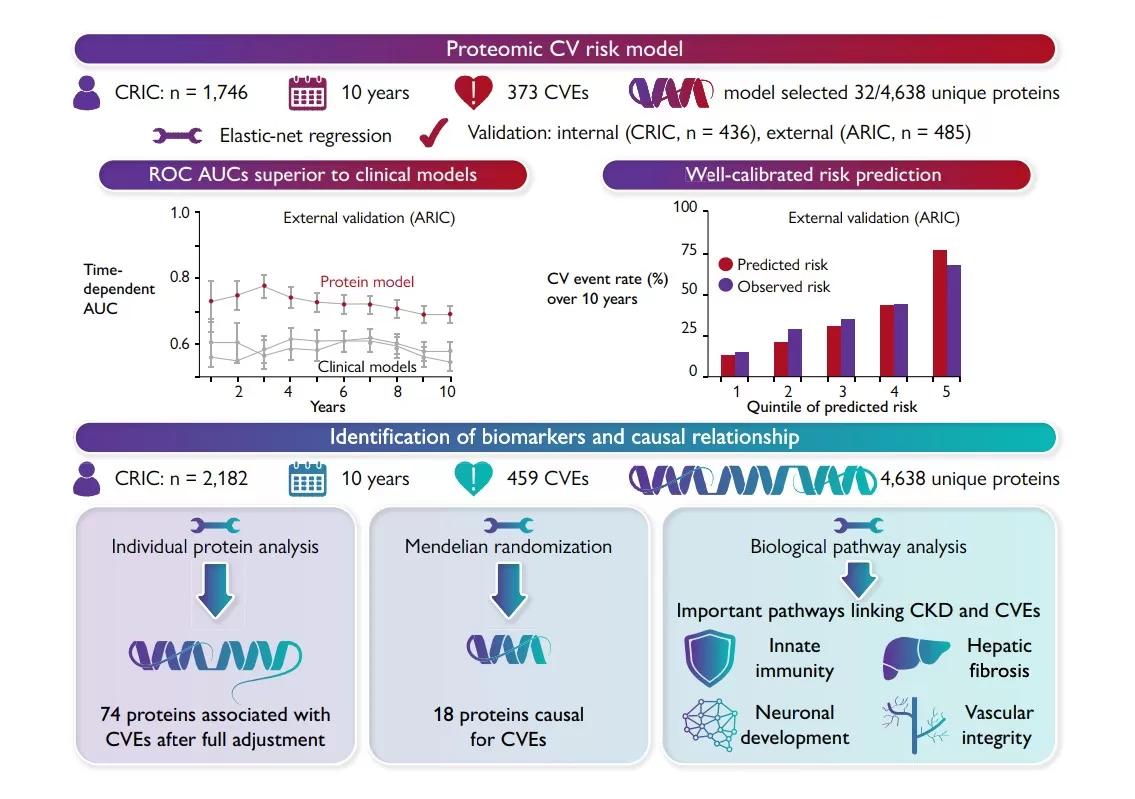
Proteomic Risk Model Workflow and Predictive Performance in CKD [18]
Reference
1. Kjærgaard J, Stocks B, Henderson J, et al. Personalized molecular signatures of insulin resistance and type 2 diabetes. Cell. 2025;188(15):4106-4122.e16. doi:10.1016/j.cell.2025.05.005
2. Abozaid YJ, Ayada I, van Kleef LA, et al. Plasma proteomic signature of fatty liver disease: The Rotterdam Study. Hepatology. 2023;78(1):284-294. doi:10.1097/HEP.0000000000000300
3. Sanyal AJ, Williams SA, Lavine JE, et al. Defining the serum proteomic signature of hepatic steatosis, inflammation, ballooning and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2023;78(4):693-703. doi:10.1016/j.jhep.2022.11.029
4. Xia J, Chen H, Wang X, et al. Sphingosine d18:1 promotes nonalcoholic steatohepatitis by inhibiting macrophage HIF-2α. Nat Commun. 2024;15(1):4755. Published 2024 Jun 4. doi:10.1038/s41467-024-48954-2
5. Whitehead A, Krause FN, Moran A, et al. Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis. Nat Commun. 2021;12(1):1905. Published 2021 Mar 26. doi:10.1038/s41467-021-22272-3
6. Zhang J, Ni Y, Qian L, et al. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv Sci (Weinh). 2021;8(16):e2100536. doi:10.1002/advs.202100536.
7. Huang Q, Qadri SF, Bian H, et al. A metabolome-derived score predicts metabolic dysfunction-associated steatohepatitis and mortality from liver disease. J Hepatol. 2025;82(5):781-793. doi:10.1016/j.jhep.2024.10.015
8. Noureddin M, Truong E, Mayo R, et al. Serum identification of at-risk MASH: The metabolomics-advanced steatohepatitis fibrosis score (MASEF). Hepatology. 2024;79(1):135-148. doi:10.1097/HEP.0000000000000542.
9. Masoodi M, Gastaldelli A, Hyötyläinen T, et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18(12):835-856. doi:10.1038/s41575-021-00502-9
10. Leung H, Long X, Ni Y, et al. Risk assessment with gut microbiome and metabolite markers in NAFLD development. Sci Transl Med. 2022;14(648):eabk0855. doi:10.1126/scitranslmed.abk0855.
11. Takeuchi T, Kubota T, Nakanishi Y, et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature. 2023;621(7978):389-395. doi:10.1038/s41586-023-06466-x.
12. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55-71. doi:10.1038/s41579-020-0433-9
13. Deng K, Xu JJ, Shen L, et al. Comparison of fecal and blood metabolome reveals inconsistent associations of the gut microbiota with cardiometabolic diseases. Nat Commun. 2023;14(1):571. Published 2023 Feb 2. doi:10.1038/s41467-023-36256-y
14. Yao P, Iona A, Pozarickij A, et al. Proteomic Analyses in Diverse Populations Improved Risk Prediction and Identified New Drug Targets for Type 2 Diabetes. Diabetes Care. 2024;47(6):1012-1019. doi:10.2337/dc23-2145.
15. Tian Y, Jellinek MJ, Mehta K, et al. Membrane phospholipid remodeling modulates nonalcoholic steatohepatitis progression by regulating mitochondrial homeostasis. Hepatology. 2024;79(4):882-897. doi:10.1097/HEP.0000000000000375
16. Pham TCP, Dollet L, Ali MS, et al. TNIK is a conserved regulator of glucose and lipid metabolism in obesity. Sci Adv. 2023;9(32):eadf7119. doi:10.1126/sciadv.adf7119
17. Bezawork-Geleta A, Devereux CJ, Keenan SN, et al. Proximity proteomics reveals a mechanism of fatty acid transfer at lipid droplet-mitochondria- endoplasmic reticulum contact sites. Nat Commun. 2025;16(1):2135. Published 2025 Mar 3. doi:10.1038/s41467-025-57405-5.
18. Deo R, Dubin RF, Ren Y, et al. Proteomic cardiovascular risk assessment in chronic kidney disease. Eur Heart J. 2023;44(23):2095-2110. doi:10.1093/eurheartj/ehad115
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


