Comprehensive Guide to Single-Cell Sorting Methods: Flow Cytometry, Magnetic Beads, and Density Gradients
Single-cell sorting is a crucial technique in a wide range of biological and clinical research applications, including immunology, oncology, stem cell research, and personalized medicine. It plays a pivotal role in advancing single-cell multi-omics studies, such as single-cell transcriptomics, single-cell proteomics, and single-cell metabolomics, by enabling precise isolation of specific cell populations for downstream analyses. Whether you are studying immune responses, identifying rare cell types, or isolating stem cells for regenerative therapies, achieving accurate and reliable cell sorting is the first critical step to success. The method you choose will directly influence the purity, viability, and overall outcome of your experiment. In this blog, we will delve into three commonly used methods for single-cell sorting—flow cytometry, magnetic bead sorting, and density gradient centrifugation—discussing their principles, advantages, limitations, and ideal applications in the context of single-cell multi-omics research. This guide will help you select the optimal sorting technique for your specific research needs, ensuring the highest quality results in your multi-omics studies.
Flow Cytometry Sorting (FACS)
Flow cytometry is a powerful technique that leverages light scattering and fluorescence to sort individual cells with high precision. As cells flow through a laser beam, their size, granularity, and fluorescence emission are captured. This data enables the identification and characterization of specific cell populations based on the expression of surface markers. A computer algorithm processes this information in real-time, making rapid decisions about which cells to isolate, ensuring highly accurate and reproducible sorting results.
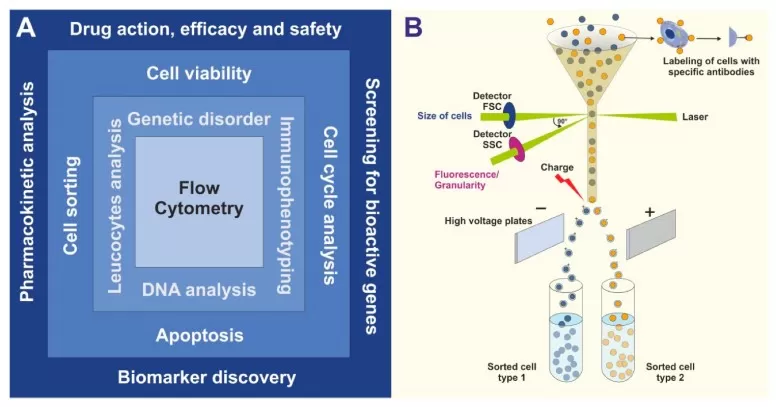
The principle of FACS analysis (image reproduced from Drescher et al., 2021)
Ideal Use Cases of Flow Cytometry Sorting:
- High-Purity Isolation: For experiments where purity is critical, flow cytometry can achieve purities of up to 99%. This is especially important when isolating cells for downstream applications like single-cell RNA sequencing (scRNA-seq) or functional assays.
- Multi-Marker Sorting: Flow cytometry excels in sorting complex populations with multiple markers (3+ colors). For example, in cancer research, you can isolate tumor-infiltrating T cells based on combinations of markers like CD45⁺CD3⁺CD8⁺PD-1⁺.
- Rare Cell Detection: This method is particularly effective for detecting rare cell populations, such as circulating tumor cells (CTCs) or hematopoietic stem cells, which might be present in low abundance.
Advantages of Flow Cytometry Sorting:
- High Purity: Flow cytometry sorting offers excellent purity of sorted cells, achieving up to 99% purity. This is crucial for experiments that require a highly purified population for downstream applications like single-cell RNA sequencing (scRNA-seq), functional assays, or gene expression analysis.
- Complex Sorting: This technique is capable of sorting cells based on multiple parameters simultaneously, making it ideal for complex experimental designs that involve multi-marker analysis. It is particularly useful when identifying and isolating rare cell populations or heterogeneous cell types.
- Cell Viability: One of the key strengths of flow cytometry sorting is that it enables live cell sorting, ensuring the viability of the sorted cells for subsequent functional studies, culture, or other assays that require active cells.
Limitations of Flow Cytometry Sorting:
- High Cell Input Requirement: To properly calibrate the machine and achieve efficient sorting, flow cytometry generally requires millions of cells. This may be a limitation when working with small sample sizes or rare populations.
- Time-Consuming: Sorting large volumes or complex samples, such as those for scRNA-seq or other high-throughput analyses, can be time-consuming, often taking hours to complete, especially when sorting multi-well plates.
- Fragile Cells: The high-pressure sheath fluid used during sorting can sometimes damage large or fragile cell types, making the method less suitable for cells that are sensitive to mechanical stress or pressure.
Magnetic Bead Sorting (MACS)
Magnetic bead sorting is a widely used technique that employs super-paramagnetic microbeads coated with antibodies specific to target cell surface antigens. The sample is passed through a magnetic column, where the beads, bound to the target cells, are captured by the magnetic field, while non-target cells are washed away. Once the magnetic field is removed, the target cells are eluted and collected for further analysis or downstream applications. This method provides a fast and efficient means of isolating specific cell populations with minimal disruption to cell viability, making it ideal for routine cell isolation and enrichment in various research fields, including immunology and stem cell biology.
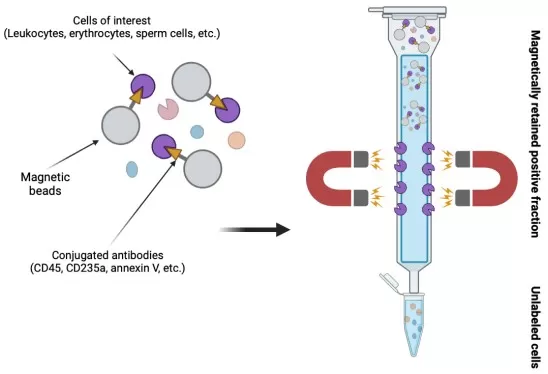
Operating principle of magnetic separation (image reproduced from Czétány et al., 2024)
Ideal Use Cases of Magnetic Bead Sorting:
- Rapid Sorting: When you need to process a large number of cells quickly, magnetic bead sorting is highly efficient, capable of handling up to 10⁹ cells in just 30 minutes.
- Moderate Purity Requirement: Magnetic bead sorting typically achieves purities of 80% to 95%, making it suitable for experiments where absolute purity is not critical. It is commonly used for isolating cell populations such as CD3⁺ T cells or CD14⁺ monocytes.
- Gentle Handling: If you need to maintain cell viability for subsequent applications like adoptive cell transfer or culturing, magnetic bead sorting is a gentle method that doesn’t expose cells to harsh conditions.
Advantages of Magnetic Bead Sorting:
- Fast and Efficient: Magnetic bead sorting is ideal for high-throughput sorting where speed is crucial.
- Gentle on Cells: The method does not require high-pressure systems, which preserves cell viability.
- Cost-Effective: Compared to flow cytometry, magnetic bead sorting is generally more affordable and doesn’t require expensive equipment.
Limitations of Magnetic Bead Sorting:
- Purity Limitations: While magnetic bead sorting is efficient, its purity generally does not exceed 95%. For higher purity, it may require additional purification steps such as flow cytometry cleanup.
- Activation of Signaling Pathways: The beads can occasionally activate cell signaling, which may interfere with certain assays. It’s important to consider this when choosing magnetic bead sorting for functional studies.
Density Gradient Centrifugation
Density gradient centrifugation is based on the principle of buoyancy, where cells are separated according to their density during centrifugation. A sample, such as blood or tissue homogenate, is carefully layered over a density gradient medium (e.g., Ficoll, Percoll, or sucrose). Upon centrifugation, cells migrate through the gradient and segregate into distinct layers based on their density. Target cells, such as peripheral blood mononuclear cells (PBMCs), typically form a distinct band at the interface between the gradient layers, while denser cells like red blood cells and platelets settle at the bottom, and lighter cell components remain near the top. This method provides a simple, label-free approach for isolating specific cell populations and is widely used for enriching PBMCs and other cell types in immunology and cell biology research.
 and non-adherent (B) breast cancer cells to isolate cancer stem cells_1765415076_WNo_641d767.webp)
Density gradient centrifugation protocols for adherent (A) and non-adherent (B) breast cancer cells to isolate cancer stem cells (image reproduced from Sargiacomo et al., 2024)
Ideal Use Cases of Density Gradient Centrifugation:
- PBMC Enrichment: Density gradient centrifugation is an efficient and reliable technique for isolating peripheral blood mononuclear cells (PBMCs) from whole blood. This method does not require antibodies or fluorescent markers, making it ideal for researchers who need a quick and straightforward cell isolation process.
- Cell Purification: This method is widely used for the purification of various cell types, including lymphocytes, monocytes, and cell nuclei. It is well-suited for a range of research applications, particularly in immunology and cell biology.
- Scalability: Density gradient centrifugation is highly scalable, capable of processing small sample volumes (as little as 1 mL) or larger volumes (up to 500 mL), making it adaptable to both small-scale and high-throughput studies.
Advantages of Density Gradient Centrifugation:
- Simple and Label-Free: Unlike other sorting techniques, density gradient centrifugation does not require antibodies, magnetic beads, or lasers, making it a great choice for researchers looking to avoid potential surface perturbation or unwanted labeling.
- Cost-Effective: This technique requires only a standard centrifuge, making it a more accessible option for labs with limited resources and budget constraints.
- Rapid Processing: PBMC enrichment can be completed in less than 20 minutes, offering a fast and efficient method for cell isolation.
Limitations of Density Gradient Centrifugation:
- Low Resolution: The method provides a less precise separation of cell types compared to other sorting techniques. It is best suited for isolating bulk populations of mononuclear cells (lymphocytes and monocytes) but may not be effective for isolating specific cell subsets with high resolution.
- Suboptimal Granulocyte Recovery: If the gradient is too harsh, granulocytes may not be efficiently recovered, which can limit its use for studies requiring a high yield of granulocytes.
- Risk of Contamination: Overloading the sample or using improper deceleration speeds during centrifugation can result in smeared bands, reducing the purity of the isolated cell populations. Careful optimization of the protocol is required to maintain purity.
Quick Decision Guide: Choosing the Right Cell Sorting Method
Selecting the optimal cell sorting method depends on your specific experimental requirements, such as cell purity, throughput, and available resources. Flow cytometry is the best option when high purity and precise multi-marker sorting are essential. This method excels at isolating complex cell populations and rare cell types with high sensitivity, making it ideal for applications like single-cell RNA sequencing and functional assays. On the other hand, magnetic bead sorting provides a rapid, cost-effective solution, particularly when working with fewer markers or when cell viability is a priority. It's a great choice for routine cell enrichment, especially when working with larger cell populations or when gentle handling is required. Density gradient centrifugation, while offering lower resolution, is perfect for larger sample sizes or situations where you want to avoid using antibodies or reagents. This method is well-suited for bulk enrichment of cells, such as PBMCs, without the need for complex equipment or labeling techniques.
By understanding the unique advantages and limitations of each technique, you can make an informed decision that aligns with your research goals. Whether isolating immune cells, rare cell populations, or PBMCs for clinical applications, choosing the right sorting method is crucial for ensuring the success of your experiments. Mastering these sorting techniques will not only streamline your workflows but also enhance the reproducibility and reliability of your research, contributing to more meaningful and impactful scientific outcomes.
Reference:
1. Drescher H, Weiskirchen S, Weiskirchen R. Flow Cytometry: A Blessing and a Curse. Biomedicines. 2021;9(11):1613. Published 2021 Nov 4. doi:10.3390/biomedicines9111613
2. Czétány P, Balló A, Márk L, Török A, Szántó Á, Máté G. An Alternative Application of Magnetic-Activated Cell Sorting: CD45 and CD235a Based Purification of Semen and Testicular Tissue Samples. Int J Mol Sci. 2024;25(7):3627. Published 2024 Mar 24. doi:10.3390/ijms25073627
3. Sargiacomo C, Klepinin A. Density Gradient Centrifugation Is an Effective Tool to Isolate Cancer Stem-like Cells from Hypoxic and Normoxia Triple-Negative Breast Cancer Models. Int J Mol Sci. 2024;25(16):8958. Published 2024 Aug 17. doi:10.3390/ijms25168958
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


