Ceramides vs Niacinamide: How They Strengthen the Skin Barrier
For R&D teams and clinical researchers, “ceramides vs niacinamide” is no longer just a cosmetic marketing debate but a question about mechanisms, readouts, and study design. Ceramides build the lamellar architecture that keeps transepidermal water loss (TEWL) low, while niacinamide boosts epidermal lipid synthesis and barrier resilience over time. Here, we connect ceramide subclasses (NP/NS, EOS/EOP), niacinamide’s NAD⁺-linked biology, and tape-stripping lipidomics to real-world skin barrier outcomes.
Ceramides in the Stratum Corneum: Subclasses, Chain Length, and Barrier Physics
What they are. Ceramides are sphingolipids composed of a sphingoid base amide-linked to a fatty acid; together with cholesterol and free fatty acids, they form highly ordered lamellae that confer low permeability [3,5]. For a deeper dive into ceramide biosynthesis and disease links, see our background explainer on Ceramide Metabolism, Lipid Signaling & Disease.
NP/NS as a functional marker. In human SC, the Cer[NP]/[NS] ratio correlates with barrier quality—higher NP (vs NS) associates with lower TEWL and higher capacitance; NP also accumulates along keratinocyte differentiation, whereas barrier-impaired skin (atopic dermatitis/psoriasis) shows NP depletion and relative NS gain [2].
ω-acylceramides EOS/EOP and the long-periodicity phase (LPP). EOS/EOP (ultra-long ω-hydroxy acyl chains esterified with linoleate) staple lipid sheets to the corneocyte envelope and stabilize the long-periodicity phase (LPP) essential for water impermeability. Their loss is characteristic of atopic skin; restore them in balanced proportions or lamellar order suffers [4,5].
Chain length matters. Barrier-competent SC is enriched in very-long-chain (C24–C26+) largely saturated ceramides; disruption or disease often shifts profiles toward shorter/unsaturated species, tracking with higher TEWL and slower recovery [3,6]. Modern lipidomics has cataloged >1,000 distinct SC ceramide species when chain permutations are considered, explaining why physiological mixtures outperform single-species add-backs [7].
 — Structures That Build the Skin Barrier_1764032976_WNo_644d259.webp)
Ceramide Subclasses (NP, NS, AP, EOS, EOP) — Structures That Build the Skin Barrier
Source: Mijaljica D, Ashbolt R, Deplazes E, et al. “Considering Phytosphingosine-Based Ceramide Formulations for Atopic Skin Care.” Dermato 2024;4(1):5–22. Figure 4. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Niacinamide Boosts Epidermal Lipid Synthesis: From NAD⁺ to SPT Upregulation
Mechanism. Niacinamide replenishes NAD⁺/NADP⁺ pools that support differentiation and lipid production. It upregulates serine palmitoyltransferase (SPT)—the rate-limiting step of sphingolipid synthesis—along with LCB1/LCB2 subunits, increasing de novo ceramides, glucosylceramides, and sphingomyelins in keratinocytes; in vivo, topical niacinamide raises SC lipid content and lowers TEWL [1,8–9].
Hydration physiology beyond lipids. Niacinamide mitigates oxidative stress and modulates aquaporin-3 (AQP3) (e.g., down-regulates retinoic-acid–induced overexpression in keratinocyte models), complementing its lipidogenic effects on barrier function [11].
Oral data. In a year-long randomized study, oral nicotinamide 500 mg twice daily produced small but consistent TEWL reductions across multiple sites (typically ~2–8%, site-dependent)—supportive of a systemic contribution to barrier resilience [10].
Ceramides vs. Niacinamide in Practice: Complementary, Not Competing
Ceramides reinforce the lamellae now, while niacinamide expands lipid-synthesis capacity over time. Used together, they deliver additive gains in transepidermal water loss (TEWL), hydration, tolerability, and even acne outcomes.
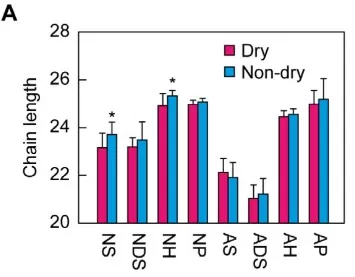
Shorter Ceramide Chains Associate with Dry Skin — LC-MS/MS Evidence
Source: Akiyama F, Takahashi N, Ueda Y, et al. “Correlations between Skin Condition Parameters and Ceramide Profiles in the Stratum Corneum of Healthy Individuals.” International Journal of Molecular Sciences 2024;25(15):8291. Figure 6A. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
The summary below compares how ceramide-dominant moisturizers and niacinamide interventions perform across study designs, TEWL and hydration readouts, and barrier recovery endpoints.
Clinical/Real-World Context at a Glance
|
Intervention |
Typical design |
Primary endpoints |
Effect size & timing |
|
Topical niacinamide (2–5%) |
3–8 weeks, controlled |
TEWL ↓; capacitance ↑; SC thickness ↑ |
Improvements commonly by week 3–4; histologic SC thickening around 4 weeks |
|
Oral nicotinamide (500 mg bid) |
6–12 months, randomized |
TEWL (multiple sites) |
Small but consistent TEWL ↓ (~2–8%, site-dependent) |
|
Ceramide-dominant emollients |
2–4 weeks; adjunct or post–tape strip |
TEWL ↓; symptom scores |
Faster barrier recovery vs control; dermatitis signs improve |
|
Niacinamide + ceramides (acne adjunct) |
8-week split-face RCT |
Lesion counts; hydration; irritation |
Greater lesion reduction than control; hydration ↑; no extra irritation |
|
Real-world cosmetics (3 weeks) |
Observational |
Corneometer hydration; redness |
NIA arms: hydration ↑; CER arms: redness ↓; both well tolerated |
Key takeaway: Ceramides give you immediate lamellar reinforcement, while niacinamide scales up the skin’s own lipid-synthesis machinery. Used together in barrier-repair protocols or tape-stripping studies, they consistently deliver larger, more durable improvements in TEWL, hydration and tolerability than either ingredient alone.
Ceramides vs Niacinamide—At a Glance
|
Aspect |
Ceramides |
Niacinamide |
|
Primary action |
Builds lamellar architecture (NP/NS balance; EOS/EOP; chain length) |
Upregulates lipid synthesis (SPT/LCB1/LCB2), supports NAD⁺ metabolism |
|
Onset |
Immediate lamellar reinforcement |
Gradual (~3–4 weeks) lipidogenesis & hydration |
|
Best use case |
Rapid barrier support; post–tape strip; dermatitis adjunct |
Daily maintenance; irritant tolerance; pairing with actives |
|
Readouts |
TEWL ↓, faster recovery; subclass/chain-length shifts |
TEWL ↓, capacitance ↑; SC thickness ↑ |
Non-Invasive Readouts That Prove It: TEWL, Tape-Strip Lipidomics, and Correlations
TEWL via evaporimetry remains the functional gold standard for barrier integrity. Tape stripping either (i) generates a controlled barrier insult to track recovery or (ii) collects SC for liquid chromatography–mass spectrometry (LC–MS/MS) lipidomics. Studies that pair longitudinal TEWL with tape-strip ceramide profiling show that shifts toward higher NP/NS, sufficient EOS/EOP, and longer chain-length distributions track with faster TEWL normalization and better hydration [6,14–15]. This closes the loop from mechanism → molecules → clinical endpoint.
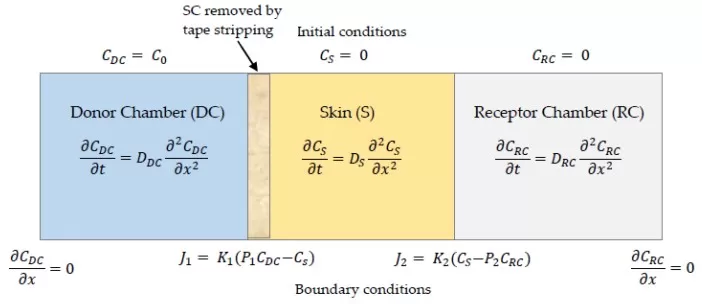
How Tape-Stripping Studies the Barrier — Franz Cell & Layered-Skin Model
Source: Gudnason H, et al. “Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling.” Pharmaceutics 2022;14(9):1880. Figure 2. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Analytical Methods: Measuring Ceramides vs. Niacinamide
Ceramides: LC–MS/MS (often multiple reaction monitoring [MRM] or PRM) is the gold standard for class/species resolution (e.g., Cer NS 24:0, EOS C36). HPTLC or HPLC-ELSD can screen formulations but lack species-level detail essential for mechanism-to-endpoint work [3,7].
Niacinamide: High-performance liquid chromatography (HPLC) with UV (~262 nm) is robust for product assays/stability; LC–MS/MS provides sensitivity and selectivity in skin/tape-strip extracts. In combined studies, split extracts and run orthogonal methods for non-polar lipids vs. polar niacinamide [16].
Limitations and Gaps
Most cosmetic/dermatologic studies remain short-term (3–8 weeks) with modest sample sizes and heterogeneous formulations/sites. ω-acylceramide restoration in atopic dermatitis is formulation-sensitive, and species-level reporting (not just totals) is still uncommon. Future trials should pre-register species-level ceramide endpoints and co-report TEWL/capacitance to strengthen causal inference.
FAQ: Ceramides, Niacinamide and Skin Barrier Research
Q1. What TEWL effect sizes are realistic for niacinamide?
Topical 2–5% commonly shows TEWL ↓ and capacitance ↑ by week 3–4; oral 500 mg bid yields small, site-dependent TEWL decreases over months [8–10].
Q2. Which ceramide features best track with barrier function?
Higher Cer[NP]/[NS], adequate EOS/EOP, and very-long-chain enrichment (C24–C26+) correlate with lower TEWL and faster recovery [2–3,6–7].
Q3. How to design a tape-strip + lipidomics study?
Standardize strip number/depth and timepoints (baseline/post-strip/days 3–14), quantify NP/NS/AP/AS/EOS/EOP with chain-length bins by LC–MS/MS, and co-measure TEWL/capacitance for molecular-functional linkage [6,14–15].
Q4. Can a moisturizer mimic the full SC ceramide diversity (>1,000 species)?
No; aim for representative classes (NP/AP/NS/AS/EOS/EOP) and physiologic ratios/chain lengths to approximate packing physics and LPP stability [5,7].
Q5. Where does AQP3 fit into barrier improvements?
Niacinamide can modulate AQP3 (e.g., down-regulating retinoic-acid–induced overexpression), but the primary drivers of TEWL reduction are lipidogenesis and lamellar organization [11].
Conclusion
Ceramides build the barrier’s architecture, while niacinamide builds the skin’s capacity to maintain it. Mechanistically, subclass composition (NP/NS, EOS/EOP) and chain-length distribution determine lamellar physics and TEWL; niacinamide turns up SPT-driven sphingolipid output and improves tolerance to irritants. Clinically, pairing the two yields additive benefits on hydration, TEWL, irritation, and acne outcomes within weeks, with tape-strip lipidomics providing the molecular evidence behind the functional readouts. For researchers and formulators, this mechanism-to-endpoint approach enables smarter study designs and more predictive product claims.
Quantitative lipidomics, made practical. If you need to quantify ceramide subclasses (NP/NS/AP/AS/EOS/EOP), track chain-length distributions, or link species-level shifts to TEWL in tape-strip studies, our Quantitative Lipidomics Service delivers targeted LC–MS/MS panels and actionable summaries—so your mechanism translates into verifiable endpoints.
References
- Tanno O, Ota Y, Kitamura N, Katsube T, Inoue S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br J Dermatol. 2000;143(3):524-531. doi:10.1046/j.1365-2133.2000.03705.x
- Yokose U, Ishikawa J, Morimoto Y, et al. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020;20(1):6. doi:10.1186/s12895-020-00102-1
- van Smeden J, Bouwstra JA. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr Probl Dermatol. 2016;49:8-26. doi:10.1159/000441540
- Mijaljica D, Fitzsimons A, Wade J, et al. Considering phytosphingosine-based ceramide formulations for atopic skin care. Dermato. 2024;4(1):5-22. doi:10.3390/dermato4010002
- van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841(3):295-313. doi:10.1016/j.bbalip.2013.11.006
- Boiten WA, Absalah S, van Smeden J, Bouwstra JA. Applying a vernix caseosa–based formulation accelerates skin barrier repair by modulating lipid biosynthesis. J Lipid Res. 2018;59(2):250-260. doi:10.1194/jlr.M079186
- van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, Bouwstra JA. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res. 2011;52(6):1211-1221. doi:10.1194/jlr.M014456
- Mohammed D, Matts PJ, Hadgraft J, Lane ME. Influence of niacinamide-containing formulations on the molecular and biophysical properties of the stratum corneum. Int J Pharm. 2013;441(1–2):192–201. doi:10.1016/j.ijpharm.2012.11.043
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76(2):135-141.
- Chen AC, Martin AJ, Dalziell RA, Halliday GM, Damian DL. Oral nicotinamide reduces transepidermal water loss: a randomized controlled trial. Br J Dermatol. 2016;175(6):1363-1365. doi:10.1111/bjd.14648
- Song S, Lee ES, Jeong GS, et al. Nicotinamide attenuates retinoic acid–induced aquaporin-3 overexpression in keratinocytes. Int J Mol Med. 2018;41(6):3509-3518. doi:10.3892/ijmm.2018.3511
- Tempark T, Pongpirul K, Lueangarun S, et al. Efficacy of ceramide- and niacinamide-containing moisturizer versus hydrophilic cream with topical anti-acne treatment in mild-to-moderate acne: a split-face randomized controlled trial. J Cosmet Dermatol. 2024;23(5):1758-1765. doi:10.1111/jocd.16212
- Załęcki P, Jezusek J, Nowicka D. Topical niacinamide in daily skincare: a 3-week real-world cosmetic study. Appl Sci. 2025;15(17):9729. doi:10.3390/app15179729
- Tsoi LC, Xing X, Xing E, et al. Noninvasive Tape-Stripping with High-Resolution RNA Profiling Effectively Captures a Preinflammatory State in Nonlesional Psoriatic Skin. J Invest Dermatol. 2022;142(6):1587-1596.e2. doi:10.1016/j.jid.2021.09.038
- Hughes AJ, Tawfik SS, Baruah KP, O'Toole EA, O'Shaughnessy RFL. Tape strips in dermatology research. Br J Dermatol. 2021;185(1):26-35. doi:10.1111/bjd.19760
- Yang Y, Strickland Z, Kapalavavi B, Marple R, Gamsky C. Industrial application of green chromatography--I. Separation and analysis of niacinamide in skincare creams using pure water as the mobile phase. Talanta. 2011;84(1):169-174. doi:10.1016/j.talanta.2010.12.044
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


