Indole: The Microbial Metabolite Connecting Gut Health, Brain, and Immunity
Indole is a small molecule produced by gut bacteria from tryptophan, long recognized for its distinct odor but now understood as a key chemical messenger between microbes and the host. It plays vital roles in modulating gut barrier function, inflammation, brain health, and tumor immunity. This blog explores indole’s origin, biosynthesis, metabolism, and its implications in major human diseases like IBD, neurodegeneration, and cancer.
Unearthing Indole: Discovery and Chemical Structure
What Is Indole? Structure, Odor Duality, and Biological Roles
Indole is a naturally occurring organic compound produced by gut bacteria through the metabolism of the amino acid tryptophan. Known for its odor duality—pungent at high concentrations yet floral at trace levels and unpleasant at high concentrations, but floral and pleasant in trace amounts — indole plays essential roles in microbiota-host communication, gut barrier function, immune regulation, and brain health. It is also found in perfumes, feces, and fermented foods, making it a molecule that bridges both biology and daily life.
History of Indole: From Indigo to Modern Biology
Indole was first obtained in 1866 by Adolf von Baeyer during the reductive/thermal decomposition of indigo (e.g., destructive distillation with zinc dust). In 1869, Baeyer proposed the structural formula—a benzene ring fused to a pyrrole ring—which cemented indole as a fundamental heteroaromatic scaffold. This breakthrough bridged dye chemistry and biological discovery, paving the way for modern insights into tryptophan metabolism, neurotransmitters, and bioactive indole alkaloids.
Chemical Structure of Indole: An Aromatic Heterocycle
Indole’s chemical structure consists of a bicyclic aromatic ring system — a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Its molecular formula is C₈H₇N. This flat, conjugated structure makes indole electron-rich and chemically reactive, especially at the 3-position, where many biological substitutions occur. Indole serves as a key scaffold in many biologically active molecules including tryptophan, serotonin, melatonin, and several indole alkaloid drugs. It is a central motif not only in microbial metabolites but also in neurotransmitters and pharmaceutical compounds.
Indole Biosynthesis in the Gut: Tryptophan to Microbial Signal
Indole is primarily synthesized by gut bacteria through the breakdown of dietary tryptophan. The key enzyme involved is tryptophanase (TnaA), encoded by the tnaA gene in many microbial species such as E. coli. This enzyme converts tryptophan into indole, pyruvate, and ammonia. Eukaryotic cells lack this pathway, making indole biosynthesis a microbial hallmark. The availability of tryptophan in the gut environment regulates this process via the bacterial tna operon.
Beyond indole itself, gut microbes also produce diverse indole derivatives like indole-3-acetic acid (IAA), indole-3-propionic acid (IPA), and indole-3-lactic acid (ILA) through parallel tryptophan metabolism routes. These derivatives engage in cross-feeding across different bacterial taxa, shaping the pool of bioactive indoles.
Importantly, indole functions as a signaling cue influencing biofilm, virulence, and stress tolerance — it serves as a quorum-sensing signal, modulating bacterial behavior including biofilm formation and virulence. Its production varies by diet and microbiota composition, positioning indole as a dynamic mediator in the gut microbiome-host interface and an actionable node for microbiome-based interventions.
Indole Metabolism: The Gut-Liver Axis and Systemic Fate
After microbial production, indole is absorbed by intestinal cells and enters the portal circulation, reaching the liver for detoxification. There, it is converted by cytochrome P450 enzymes (e.g., CYP2E1, CYP2A6) into indoxyl, which is then sulfated to form indoxyl sulfate, a major circulating indole metabolite. Indoxyl sulfate is excreted by the kidneys but can accumulate in renal dysfunction, acting as a uremic toxin associated with oxidative stress and inflammation.
Other metabolic routes include methylation to skatole (3-methylindole) or conjugation with glucuronide or amino acids. These reactions reflect how the body treats indole like a xenobiotic compound, enhancing solubility for clearance.
Indole metabolism also intersects with the host’s kynurenine pathway, which dominates tryptophan degradation. The balance between microbial indole production and host kynurenine metabolism is increasingly recognized as critical for immune modulation and metabolic health. Disruption in this balance has been linked to chronic diseases, making indole a valuable marker in gut-liver axis research and clinical metabolomics.
Indole and Inflammatory Bowel Disease (IBD): A Gut Microbial Shield
Indole and its microbial derivatives play a pivotal role in gut immune homeostasis, making them highly relevant in inflammatory bowel disease (IBD) such as Crohn’s disease and ulcerative colitis. These metabolites, particularly indole-3-propionic acid (IPA) and indole-3-aldehyde (IAld), are produced by gut bacteria through tryptophan metabolism. They activate the aryl hydrocarbon receptor (AhR) in intestinal epithelial and immune cells, promoting tight junction protein expression, mucus layer integrity, and anti-inflammatory cytokines like IL-22 and IL-10.
In IBD patients, reduced levels of indole-producing bacteria have been consistently observed, correlating with increased intestinal permeability (“leaky gut”) and dysregulated immune responses. A high-impact study (Lamas et al., Nat Med, 2016) showed that CARD9-deficient mice failed to convert tryptophan into AhR ligands, leading to worsened colitis; supplementation with IAld restored mucosal integrity. Furthermore, indole derivatives like indole-3-lactic acid (ILA) from Lactobacillus species have shown protective effects in murine colitis models by enhancing beneficial cross-feeding and AhR signaling.
These findings suggest that restoring indole pathways through diet, probiotics, or postbiotics may offer a therapeutic strategy for managing IBD by stabilizing the gut microbiome-immune interface.
Indole in Neuroinflammation and Neurodegeneration
Microbial tryptophan catabolites—including indole-3-propionic acid (IPA) and, in model-dependent fashion, IAA/ILA—can influence the CNS by engaging AhR in microglia and astrocytes, thereby dampening NF-κB/NLRP3-driven neuroinflammation and oxidative stress. In Alzheimer’s disease models, mixtures enriched in indole derivatives associate with lower amyloid burden, decreased TNF-α/IL-1β, and improved cognition, aligning with a gut–brain axis mechanism. While IPA shows the most consistent evidence for brain exposure, the CNS pharmacology of other indoles likely depends on dose, matrix, and microbiome context. These observations support microbiome-targeted strategies to elevate beneficial indole signals as adjuncts to neurodegeneration therapies.
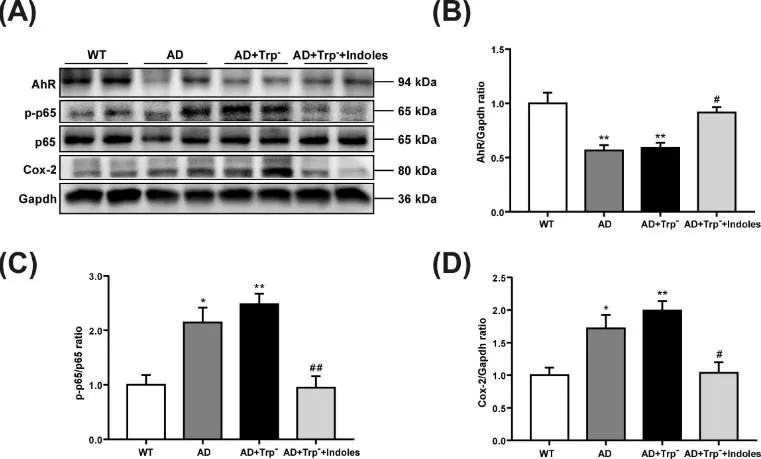
Indole Metabolites Modulate Neuroinflammation in Alzheimer’s Disease
Source: Adapted from Sun J, Wang F, Hu X, et al. Brain Behav Immun. 2022;106:76-88. doi:10.1016/j.bbi.2022.08.003. Licensed under CC BY 4.0.
Indole and Cancer: Immune Modulator with Dual Faces
Indole-derived metabolites exert both pro- and anti-tumor effects, making them key players in the tumor microenvironment (TME). On one hand, compounds such as indole-3-acetic acid (IAA) and indole activate AhR in tumor-associated macrophages (TAMs), promoting an M2-like immunosuppressive phenotype that hinders CD8⁺ T-cell activity. This immune dampening facilitates tumor progression and has been linked to poor prognosis in several cancers.
A 2022 study (Hezaveh et al., Immunity) demonstrated that indole-producing gut microbes exacerbate tumor growth in pancreatic cancer by sustaining suppressive TAMs. Conversely, some indole derivatives, such as indole-3-aldehyde (IAld), have shown immune-activating potential by stimulating IFN-γ production from CD8⁺ T cells and enhancing dendritic cell function. Additionally, modulating gut microbiota composition to favor beneficial indole producers may improve responsiveness to immune checkpoint inhibitors.
The net effect of indole in cancer depends on tumor type, microbial context, and host immune tone. Understanding and selectively steering these metabolic pathways may offer novel strategies in cancer immunotherapy, transforming indole from a double-edged sword into a clinical ally.
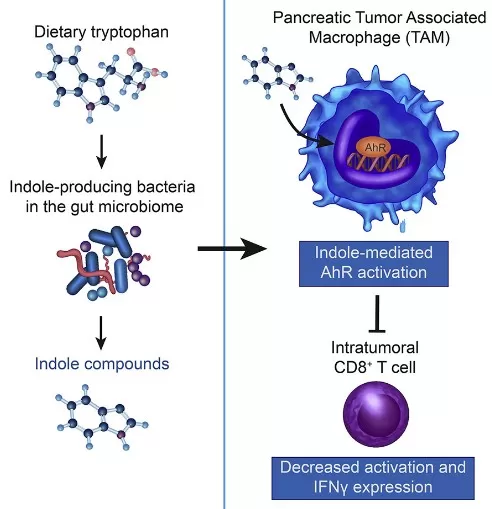
Microbial Indole Derivatives Regulate Tumor Immune Microenvironment via AhR
Source: Adapted from Kebria Hezaveh K, Li X, Mahapatra S, et al. Immunity. 2022;55(2):324-340.e8. doi:10.1016/j.immuni.2022.01.006. Licensed under CC BY 4.0.
Indole in Daily Life: Where Gut Chemistry Meets Everyday Experience
Indole connects microbial metabolism to daily life in surprising ways. At high concentrations, it smells like feces; at low levels, it’s found in floral perfumes. This duality also manifests in foods: many fermented foods—like aged cheese, soy sauce, and stinky tofu—derive their distinct aroma from indole produced by bacteria breaking down tryptophan. In the gut, diets rich in protein or fiber fuel indole production by commensal microbes, influencing gut barrier integrity and immune function.
Emerging evidence links indole to gut-brain signaling, where it may reduce inflammation and modulate mood—prompting interest in psychobiotics and postbiotic supplements. For example, indole-3-propionic acid (IPA) is being explored for neuroprotection and anti-inflammatory effects. Indole derivatives are also used in biomarker panels to assess gut microbiota health and metabolic balance. From nutrition to cognition, indole exemplifies how microbial metabolites influence daily wellbeing—and may shape future functional foods and microbiome-based therapies.
How to Measure Indole and Indole Derivatives
|
Analytical goal |
Typical matrix |
Preferred method |
Notes |
|
Indole, skatole quantification |
Stool, serum, plasma |
LC–MS/MS (MRM) |
Sensitive; requires anti-oxidation handling; lower LOQs |
|
Indoxyl sulfate |
Serum, plasma, urine |
Protein binding consideration; uremic toxin biomarker |
|
|
IPA, IAA, ILA panel |
Serum, CSF (select), stool |
Stable-isotope internal standards recommended |
|
|
Discovery of novel indoles |
Multimatrix |
Untargeted LC-HRMS |
Data-dependent acquisition; feature annotation; MS/MS libraries |
MetwareBio Solutions for Indole & Tryptophan Pathway Profiling
Indole’s far-reaching effects – spanning gut health, immunity, and neurology – underscore why it has become a focus in medical research. To translate these findings into health interventions, scientists and clinicians need robust ways to measure and monitor indole and its myriad metabolites. This is where cutting-edge metabolomics comes in. By profiling metabolites in blood, stool, or tissues, researchers can get a fingerprint of a person’s tryptophan metabolism: how much indole their gut is making, how it’s being processed, and how this correlates with disease states or treatment responses.
At MetwareBio, we offer a comprehensive metabolomics platform capable of accurately profiling indole and related tryptophan metabolites across diverse biological samples. Whether you're studying microbiome-host interactions, immune modulation, or biomarker discovery, our high-resolution, high-throughput system supports your research with precision and flexibility.
FAQs
Q1. What is the difference between indole and indoxyl sulfate?
Indole is a microbial product from tryptophan; indoxyl sulfate is its hepatic oxidation + sulfation product and a uremic toxin in CKD.
Q2. Does indole cross the blood–brain barrier?
IPA shows the most consistent CNS exposure; other indoles show model-dependent brain availability.
Q3. How do diet and fiber affect indole levels?
Protein and fermentable fibers modulate microbial tryptophan flux, shifting indole/IPA/IAA balance and indoxyl sulfate burden.
Q4. Best method to quantify indole derivatives?
Targeted LC–MS/MS with stable-isotope IS across serum/plasma/urine/stool; untargeted HRMS for discovery.
Q5. Are indoles good or bad for cancer immunity?
Context-dependent: some AhR signals in TAMs suppress CD8⁺ T cells; others (e.g., IAld) can enhance anti-tumor responses.
References
- Lamas B, et al. Card9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nature Medicine. 2016;22(6):598-605. doi:10.1038/nm.4102
- Sun J, et al. Microbiota-derived metabolite indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain Behav Immun. 2022;106:76-88. doi:10.1016/j.bbi.2022.08.003
- Kebria Hezaveh K, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55(2):324-340.e8. doi:10.1016/j.immuni.2022.01.006
Read more
- Malic Acid vs. Citric Acid: The Powerhouse Acids in Your Favorite Fruits
- Fumaric Acid Unveiled: From Nature's Palette to Therapeutic Potential
- Pyruvic Acid: A Key Player in Cellular Metabolism and Health
- Lactic Acid: Key Roles in Human Metabolism, Diseases, and Health Implications
- Cholic Acid: The Essential Bile Acid Impacting Digestion and Health
- Kynurenine: The Hidden Metabolite Linking Immunity, Mental Health, and Disease Prevention
- Understanding Glycine: Its Metabolism and Vital Role in Human Well-Being
- Leucine: The Branched-Chain Amino Acid That Fuels Muscle Growth
- Unveiling Ornithine: Beyond the Urea Cycle, A Multifaceted Player in Health
- Tryptophan: Essential Amino Acid for Mood, Sleep, and More
- Phenylalanine: Essential Roles, Metabolism, and Health Impacts
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


