Isobutyric Acid (Isobutyrate): An Overlooked Branched SCFA in Gut Health & Disease
Isobutyric acid (2-methylpropanoic acid) is a branched-chain short-chain fatty acid (BCFA) that predominantly appears as isobutyrate in biological samples. Although a minor gut microbial metabolite, it is a readout of protein fermentation—complementary to fiber-derived butyrate. This article clarifies how isobutyrate differs from butyrate, how gut microbes produce it from valine, what it can (and cannot) tell us in IBD, metabolic health, NAFLD/NASH, and CRC, and how to measure and interpret BCFA data (GC–MS vs LC–MS/MS, absolute vs relative values, and key confounders).
Isobutyrate quick sheet—key physicochemical and biological attributes.
|
Metabolite |
Isobutyric Acid (Isobutyrate) |
|
IUPAC Name |
2-Methylpropanoic acid |
|
Formula |
C₄H₈O₂ |
|
Molar Mass |
88.11 g/mol |
|
pK_a |
~4.8 (carboxylic acid) |
|
SCFA Class |
Branched-chain short-chain fatty acid (BCFA) |
|
Microbial Source |
Protein (valine) fermentation |
Isobutyric Acid (Isobutyrate): Definition, Source, and Why It Matters
What is isobutyric acid (isobutyrate)? Isobutyric acid (2-methylpropanoic acid) is a branched-chain short-chain fatty acid (BCFA) primarily produced when gut microbes ferment the BCAA valine. In biology it occurs mostly as isobutyrate, the conjugate base. Unlike butyrate, which is generated from dietary-fiber fermentation and fuels colonocytes, isobutyrate is best viewed as a readout of protein fermentation—useful for interpreting diet–microbiome balance rather than as a stand-alone effector. In stool or plasma panels, elevated isobutyrate often accompanies high-protein, low-fiber patterns and slow transit, whereas fiber-rich diets favor butyrate. Emerging studies link BCFA profiles to inflammation, metabolic traits, and liver health, but findings are context-dependent and influenced by sample type and covariates (diet, Bristol stool scale, medications). Practically, tracking absolute concentrations alongside ratios (e.g., butyrate:isobutyrate) helps distinguish shifts in substrate use from true changes in production. In short, isobutyrate is a microbial-metabolic marker that adds interpretive power to SCFA panels when reported with proper context.
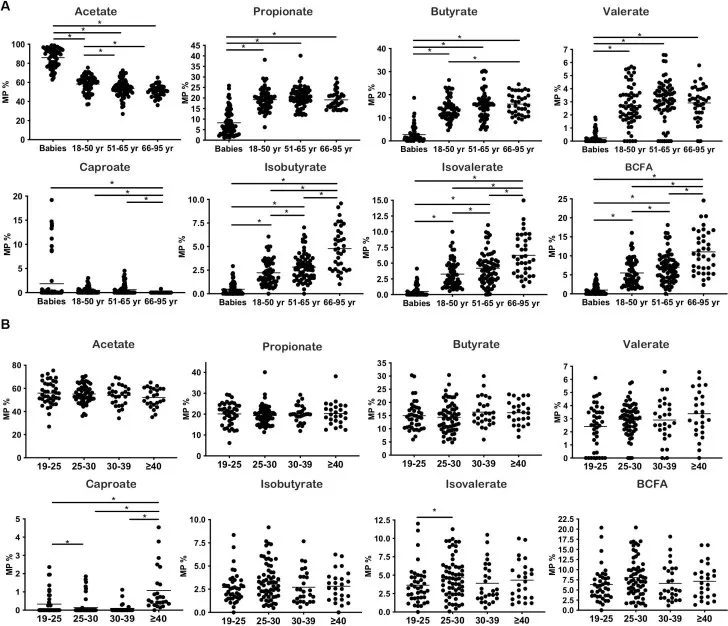
Molar proportions of SCFAs/BCFAs across lifespan and BMI; BCFA (incl. isobutyrate) trends increase with age; BMI patterns shown. (Source:Ríos-Covián D et al., Frontiers in Microbiology (2020), Figure 1, CC BY 4.0. No endorsement implied.)
History & Nomenclature: From Flavor Compound to Microbiome Marker
Identified in 19th-century studies of volatile fatty acids, isobutyric acid was initially noted for a sharp, “cheesy/rancid” aroma and discussed mainly as a flavor/off-flavor molecule in fermented foods. The name “butyric” derives from butyrum (butter), with the “iso-” prefix reflecting the branched isomer relative to straight-chain butyric acid. For much of the 20th century, its biological relevance remained unclear. The turning point came with microbiome and metabolomics research, which consistently detected isobutyrate as a minor fecal SCFA and tied it to amino-acid/protein fermentation—particularly from valine—in contrast to the carbohydrate-derived major SCFAs (acetate, propionate, butyrate). As analytical workflows matured (GC–MS for stool; LC–MS/MS with 3-NPH derivatization for low-abundance matrices), isobutyrate shifted from flavor chemistry to a biomarker that helps interpret dietary patterns, microbial ecology, and disease contexts. Today it is commonly reported in SCFA panels alongside isovalerate and 2-methylbutyrate and is increasingly incorporated into multi-omics signatures of gut health.
Isobutyric Acid vs. Butyric Acid: Structure, Origins, Roles
Despite sharing C₄H₈O₂, isobutyric acid and butyric acid diverge in structure, substrate origin, and physiology. Butyrate is mainly fiber-derived and colonocyte-fueling; isobutyrate is valine-derived and most informative as a protein-fermentation marker—useful for interpreting diet–microbiome balance rather than as a direct effector.
Key Differences:
|
Feature |
Butyric Acid (Butyrate) |
Isobutyric Acid (Isobutyrate) |
|
Structure |
Straight-chain (butanoate) |
Branched (2-methylpropanoate) |
|
Main substrate |
Dietary fiber fermentation |
Valine (BCAA) fermentation |
|
Typical producers |
Faecalibacterium, Roseburia |
Proteolytic taxa (e.g., Clostridia) |
|
Abundance |
Major SCFA |
Minor BCFA |
|
Physiological role |
Colonocyte fuel; anti-inflammatory (HDACi) |
Biomarker of proteolysis; bioactivity unclear |
|
Dietary signature |
High-fiber ↑ |
High-protein / low-fiber ↑ |
Tracking both acids in microbiome or metabolomics studies offers complementary insights into fiber- vs protein-driven fermentation patterns—critical for interpreting gut health and dietary effects.
How Do Gut Microbes Produce Isobutyrate? Metabolic path: Valine → isobutyryl-CoA → isobutyrate
Gut microbes primarily produce isobutyrate through fermentation of valine, a branched-chain amino acid (BCAA). In the anaerobic colon, undigested protein residues undergo microbial proteolysis, leading to catabolic pathways like valine → isobutyryl-CoA → isobutyrate. This process occurs alongside ammonia and CO₂ release, forming part of the gut’s protein fermentation profile.
Unlike carbohydrate fermentation (which yields SCFAs like butyrate and acetate), protein fermentation produces branched SCFAs or BCFAs—including isobutyrate, isovalerate, and 2-methylbutyrate. Microbial groups such as Clostridium and Parabacteroides species shift toward amino acid metabolism when fiber is scarce. Diet strongly influences this: high-protein, low-fiber diets promote isobutyrate production, while fiber-rich diets favor butyrate.
Importantly, isobutyrate is not inherently harmful. While long associated with “putrefaction,” studies show some commensals use this pathway under normal conditions, and certain strains may even confer anti-inflammatory effects. Thus, isobutyrate production reflects microbial flexibility and substrate availability more than a disease marker per se.
Isobutyrate in Gut Inflammation & IBD
Isobutyrate is increasingly explored in IBD as a marker of altered fermentation—particularly when butyrate is low. In Crohn’s disease and ulcerative colitis, gut dysbiosis often leads to reduced fiber-fermenting bacteria and lower butyrate, while BCFAs like isobutyrate may rise due to increased protein fermentation. Some studies report higher isobutyrate in IBD stools, others show inconsistent trends—likely due to diet, inflammation status, and sample type.
Importantly, recent data suggest isobutyrate may have context-specific benefits. One mouse study during exclusive enteral nutrition reported higher isobutyrate alongside lower inflammation, suggesting a potential benefit in that context; the mechanism remains uncertain and warrants further validation.Thus, isobutyrate’s role may depend on microbial context and co-metabolites.
Still, a high isobutyrate-to-butyrate ratio is often interpreted as a dysbiotic signature, especially if fiber intake is low. For researchers, measuring a full SCFA panel and capturing dietary/clinical metadata is key to interpreting BCFAs in IBD.
Isobutyrate in Metabolic Health: Obesity & Insulin Resistance
Isobutyrate has emerged as a potential biomarker in metabolic health. In a large U.S. cohort (MILES), individuals with higher plasma BCFAs—including isobutyrate—had significantly lower odds of dysglycemia, even after adjusting for diet and BMI. This suggests isobutyrate may signal a metabolically favorable microbiome.
However, other studies point to risks. A study in gestational diabetes found that higher isobutyrate (and lower butyrate) in mid-pregnancy predicted increased GDM risk, suggesting that a high-protein, low-fiber microbiome signature might compromise glucose control.
Overall, context matters: isobutyrate may reflect either metabolic resilience or dietary imbalance. Researchers should consider sample type (feces vs plasma), background diet, and co-metabolites. It’s likely that isobutyrate is not a direct actor but a mirror of microbial activity shaped by diet.
Isobutyrate and the Liver: NAFLD/NASH on the Gut–Liver Axis
In NAFLD and NASH, the gut–liver axis plays a pivotal role—and isobutyrate is part of the conversation. Studies have found higher fecal isobutyrate in NAFLD patients, possibly due to increased protein fermentation and reduced butyrate-producing microbes.
Though isobutyrate's direct hepatic effects remain unclear, it may contribute indirectly. When butyrate is low and isobutyrate is high, the liver may receive fewer anti-inflammatory signals and more nitrogenous load via portal blood. This imbalance could stress the liver’s metabolic capacity.
Multi-omics analyses increasingly include isobutyrate in signatures predicting NAFLD. While it may not be causal, its presence flags a shift away from healthy fermentation patterns. For researchers, tracking SCFA ratios—especially butyrate:isobutyrate—can help evaluate microbiome–liver crosstalk.
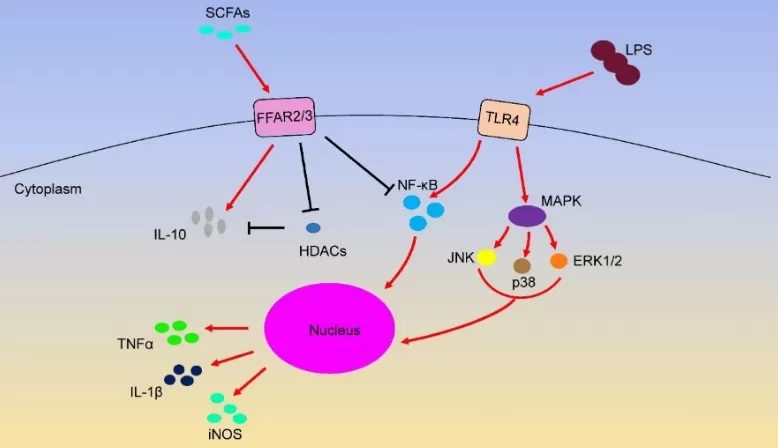
scfa signaling ffar2 ffar3 tlr4 (Source: International Journal of Molecular Sciences (2020), SCFA signalling review, figure reproduced under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.)
Isobutyrate in Colorectal Cancer Research
Unlike butyrate, which has known anti-tumor properties, isobutyrate appears neutral or even harmful in CRC. Metabolomics studies consistently show higher fecal isobutyrate in CRC patients, alongside reduced butyrate—a sign of microbial proteolysis dominating over fiber fermentation.
Preclinical signals link higher isobutyrate contexts with unfavorable CRC phenotypes, but causality and mechanisms remain unresolved and require controlled studies.
In practice, isobutyrate may serve as part of a metabolic signature for early CRC detection. A low butyrate/high isobutyrate ratio often reflects a high-protein, low-fiber gut—an environment less protective against cancer. Future interventions aiming to restore butyrate levels may shift this balance.
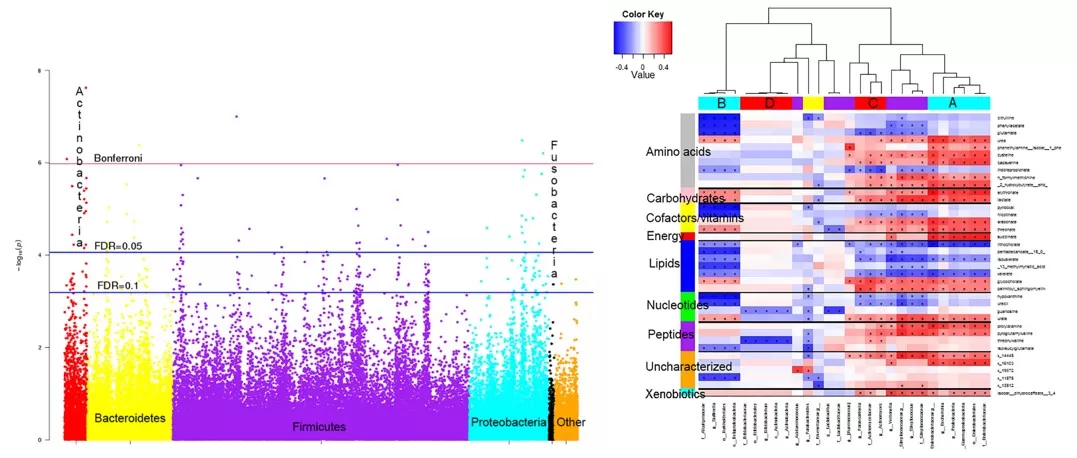
crc microbiome metabolome associations (Source:PLOS ONE (2016), Fig 1–2, CC BY 4.0. No endorsement implied.)
How to Measure Isobutyrate (Isobutyric Acid): GC–MS vs. LC–MS/MS
Accurate isobutyrate measurement is essential for microbiome and metabolomics research. The two main techniques used are GC–MS (gas chromatography–mass spectrometry) and LC–MS/MS (liquid chromatography–tandem MS), both relying on derivatization to improve detection. Read more at LC-MS VS GC-MS: What's the Difference
GC–MS is widely used for stool SCFAs, where concentrations are relatively high. Derivatization agents like PFBBr or silylation reagents improve volatility and detectability. GC–MS offers strong isomer separation and is cost-effective, but sample prep and run times can be longer.
LC–MS/MS has gained traction, especially for blood or plasma samples, where isobutyrate levels are low. Derivatization with 3-NPH or O-BHA boosts ionization and chromatographic retention, allowing sensitive detection. LC–MS/MS also enables higher throughput and better multiplexing with other metabolites.
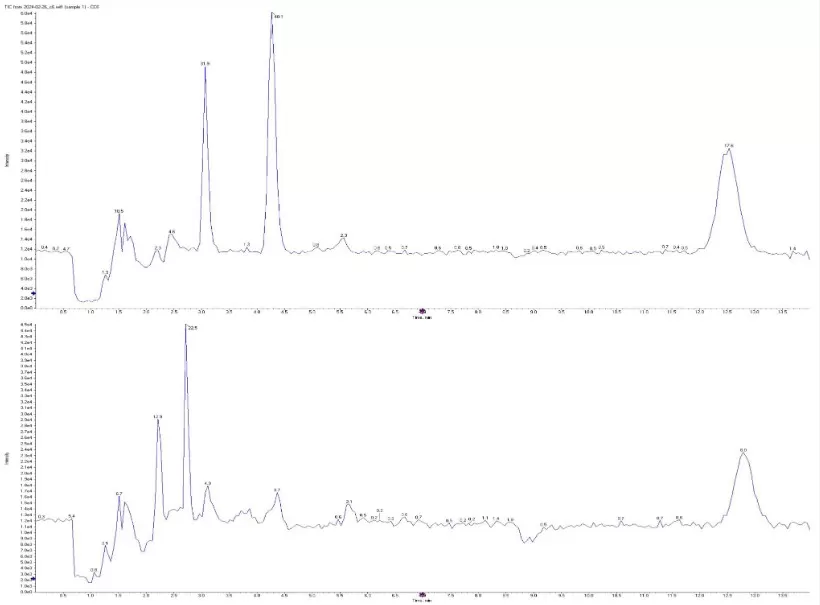
scfa chromatograms isobutyrate butyryate (Source:Metabolites (MDPI) 2024, “Plasmatic SCFAs…” Figure 1, CC BY 4.0. No endorsement implied.)
A key concern is distinguishing isobutyrate from butyrate, since they are structural isomers. With appropriate derivatization and chromatography, both platforms can resolve isobutyrate from butyrate; method optimization and matrix effects still matter. Including internal standards (e.g. labeled isobutyrate) ensures accuracy.
In summary, GC–MS is ideal for high-abundance matrices (e.g., feces), while LC–MS/MS is preferred for low-abundance samples (e.g., plasma). We run both GC–MS and LC–MS/MS in-house; for short-chain fatty acids (including branched SCFAs), our validated routine platform is GC–MS.
Pitfalls & Good Practices When Interpreting BCFA Data
Interpreting isobutyrate levels requires context. A common pitfall is relying solely on relative abundance. Since isobutyrate often rises when butyrate drops, its percentage may look high even if absolute levels are unchanged. Always report both absolute and relative concentrations.
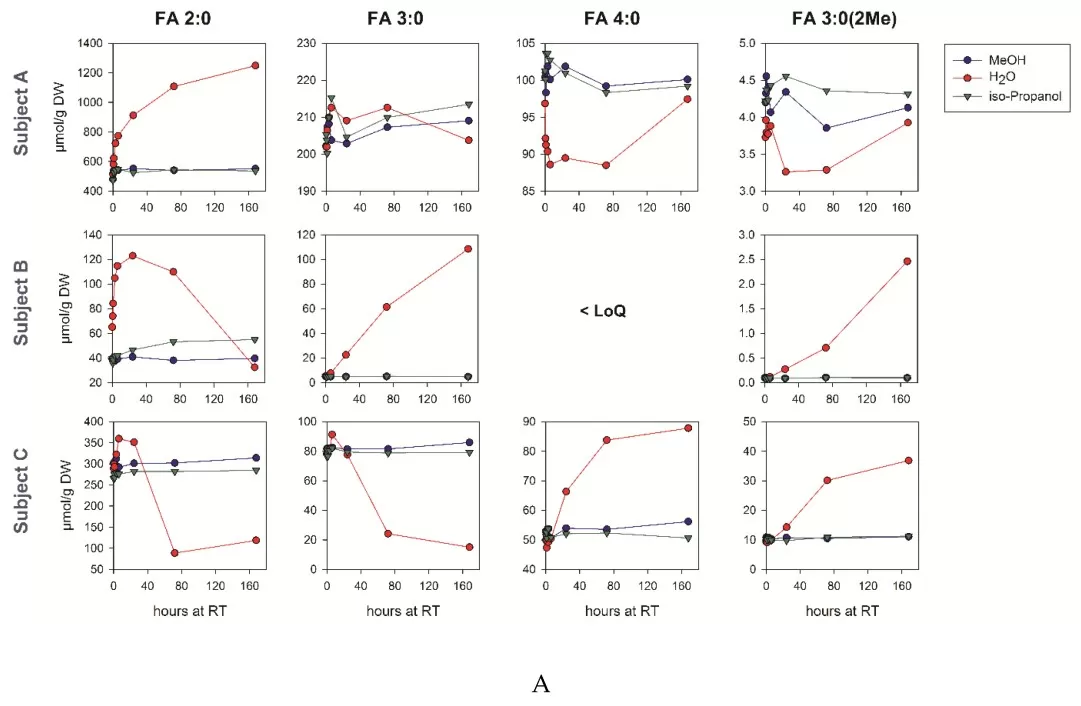
scfa preanalytical stability (Source: Biomolecules (MDPI) 2019, Figure 2, CC BY 4.0. No endorsement implied.)
Dietary intake is a major confounder—high-protein diets elevate BCFAs, while high-fiber diets favor butyrate. Without controlling or recording dietary patterns, differences in isobutyrate may reflect diet, not disease.
Gut transit time also shapes SCFA profiles: slow transit promotes protein fermentation and BCFA accumulation. Researchers should collect stool form or transit markers to help interpret findings.
Another key issue is overstating causality. Isobutyrate levels may associate with disease but aren’t necessarily causal. Studies should avoid terms like “harmful” or “protective” without mechanistic evidence.
Best practices:
- Report absolute values and ratios together
- Collect diet and stool consistency data
- Avoid drawing causal conclusions from correlations
- Use internal standards and standardized units (e.g., µmol/g)
When contextualized properly, isobutyrate can be a valuable readout of microbial function and diet–microbiome interaction, rather than a standalone biomarker.
MetwareBio: Targeted SCFA Profiling for Research
Understanding isobutyrate’s role requires accurate, contextual measurement. From protein fermentation to gut–liver signaling, this BCFA is a valuable marker in microbiome studies when interpreted with care.
MetwareBio SCFA Profiling: We provide GC–MS-based targeted SCFA quantification (stool, plasma/serum) with isomer-level separation and absolute units. For LC–MS/MS of ultra-low matrices or customized 3-NPH workflows, contact us for feasibility.
FAQs
Q1: Is isobutyrate an SCFA or a BCFA?
Both—it’s a branched-chain short-chain fatty acid (BCFA).
Q2: What diets raise isobutyrate?
A: High-protein, low-fiber patterns typically increase BCFA proportions.
Q3: Best way to measure isobutyrate?
A: GC–MS for stool (high abundance); LC–MS/MS + 3-NPH for plasma/serum (low abundance).
Q4: Is higher isobutyrate “good” or “bad”?
A: It’s context-dependent and mainly indicates protein fermentation rather than a direct effect.
Q5: Key difference vs butyrate?
A: Fiber-derived butyrate has established host benefits; valine-derived isobutyrate is best used as a biomarker of proteolysis.
References
- Wishart DS, Guo A, Oler E, et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022;50(D1):D622–D631.
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67–72.
- Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition. Nat Microbiol. 2016;1:16066.
- Beaumont M, Portune KJ, Steuer N, et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized trial in overweight humans. Am J Clin Nutr. 2017;106(4):1005–1019.
- Han J, Lin K, Sequeira C, Borchers CH. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal Chim Acta. 2015;854:86–94.
- He L, Prodhan MAI, Yuan F, et al. Simultaneous quantification of straight- and branched-chain short-chain fatty acids by GC–MS. J Chromatogr B. 2018;1092:359–367.
- da Silva HE, Teterina A, Comelli EM, et al. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8:1466.
- Le Gall G, Guttula K, Kellingray L, et al. Metabolite quantification of fecal extracts from colorectal cancer patients and healthy controls. Oncotarget. 2018;9:33278–33289.
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345.
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJM. Review article: the role of butyrate on colonic function. Gut. 2008;57(6):751–761.
- Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41.
- Ríos-Covián D, González S, Nogacka AM, Arboleya S, Salazar N, Gueimonde M, de los Reyes-Gavilán CG. An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: associated dietary and anthropometric factors. Front Microbiol. 2020;11:973.
- Vagaggini C, Brai A, Bonente D, Lombardi J, Poggialini F, Pasqualini C, Barone V, Nicoletti C, Bertelli E, Dreassi E. Development and validation of derivatization-based LC–MS/MS method for quantification of short-chain fatty acids in human, rat, and mouse plasma. J Pharm Biomed Anal. 2023;235:115599.
- Saha S, Day-Walsh P, Shehata E, Kroon PA. Development and validation of a LC–MS/MS technique for the analysis of short-chain fatty acids in tissues and biological fluids without derivatisation using isotope labelled internal standards. Molecules. 2021;26:6444.
- Furuhashi T, Ishihara G, Sugitate K. GC/MS detection of short-chain fatty acids from mammalian feces using automated sample preparation in aqueous solution. Agilent Technologies Application Note 5991-9103EN. 2019.
- Shimadzu Corporation. LC/MS/MS Method Package for Short Chain Fatty Acids (3-NPH derivatives). Technical Brochure. 2020.
Read more
- Malic Acid vs. Citric Acid: The Powerhouse Acids in Your Favorite Fruits
- Fumaric Acid Unveiled: From Nature's Palette to Therapeutic Potential
- Pyruvic Acid: A Key Player in Cellular Metabolism and Health
- Lactic Acid: Key Roles in Human Metabolism, Diseases, and Health Implications
- Cholic Acid: The Essential Bile Acid Impacting Digestion and Health
- Kynurenine: The Hidden Metabolite Linking Immunity, Mental Health, and Disease Prevention
- Understanding Glycine: Its Metabolism and Vital Role in Human Well-Being
- Leucine: The Branched-Chain Amino Acid That Fuels Muscle Growth
- Unveiling Ornithine: Beyond the Urea Cycle, A Multifaceted Player in Health
- Tryptophan: Essential Amino Acid for Mood, Sleep, and More
- Phenylalanine: Essential Roles, Metabolism, and Health Impacts
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


