Metaproteomics Guide: Turning Microbiome Proteins into Functional Insights
Metaproteomics is an emerging discipline that integrates proteomic technologies with microbiome research, focusing on the analysis of all proteins expressed within complex microbial communities. Unlike traditional microbiological methods, metaproteomics directly captures the real-time functional states and metabolic activities of microbial populations, providing a unique perspective on the interactions between microbes, their hosts, and the environment.
As microbiome research progresses, it has become clear that simply identifying the genetic composition of microbes through metagenomics is not sufficient. What is equally important is determining which proteins are actually expressed under specific environmental conditions. This is where metaproteomics holds unique value—it bridges the critical gap between genetic potential and functional expression.
What Is Metaproteomics?
Metaproteomics is dedicated to the systematic study of protein composition, abundance, modifications, and functions within complex microbial communities. Unlike culture-based approaches, it directly extracts and analyzes proteins from environmental samples such as gut contents, soil, or aquatic systems. This approach enables the simultaneous detection of thousands of proteins, thereby reflecting the true functional state of microbial communities.
Through metaproteomic analyses, researchers can identify which microbial species are actively expressing proteins and how these proteins cooperate to sustain community function. Such functional insights are critical for understanding the roles of microbial communities in both health and disease.
Metaproteomics Workflow: From Sample Collection to Data Analysis
The general workflow of metaproteomics consists of: experimental design—sample collection, preservation, and preprocessing—protein extraction—mass spectrometry data acquisition—bioinformatics analysis.
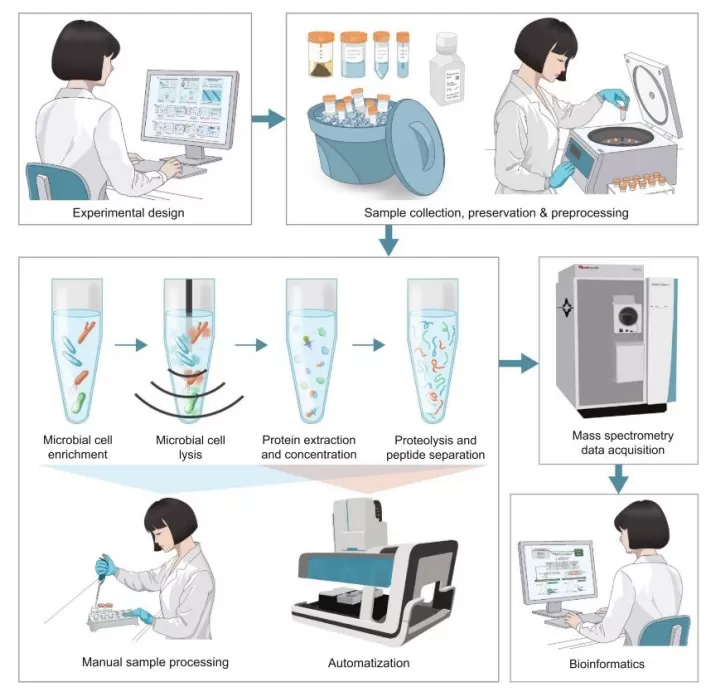
Metaproteomics Workflow
Image source: Van Den Bossche T., et al. (2025). Imeta, May 6; 4(3):e70031. doi: 10.1002/imt2.70031.
Sample Collection and Preservation
Microbiome samples are typically collected directly into sterile tubes or containers. This approach is suitable for non-invasive clinical samples such as feces, saliva, sputum, and urine, which participants can often collect themselves. For surface sampling, swabs, scrapers, or syringes are commonly used for oral, nasal, and cervicovaginal samples, while periodontal curettes or paper strips are employed for dental and gingival microbiomes. Invasive procedures, such as bronchoalveolar lavage, endotracheal aspiration, intestinal biopsy, colonic lumen aspirates, or surgical collection of colonic contents, along with gastrostomy or autopsy sampling, are applied in studies involving laboratory or wild animals. Environmental samples, by contrast, often require specialized equipment, such as quartz filters for bioaerosols or large-volume water filtration systems for aquatic environments. The choice of sampling method strongly influences the resulting metaproteomic profile, including the proportion of microbial to non-microbial components and the relative abundance of microbial taxa. Sampling strategies may also introduce operator-dependent variability, making user-friendly devices particularly valuable for clinical specimens.
Proper storage is equally essential for maintaining protein integrity and ensuring reliable downstream analysis. Environmental exposure to oxygen, temperature fluctuations, or nutrient depletion can significantly alter protein profiles. The standard preservation method for metaproteomic samples involves rapid freezing in liquid nitrogen followed by storage at –80 °C, which minimizes degradation and stabilizes protein abundance.
Protein Extraction and Preparation
Sample preparation is a pivotal first step in metaproteomic research. Extracting proteins from complex samples requires carefully optimized lysis protocols to maximize protein recovery while minimizing degradation. Common strategies combine mechanical disruption, chemical lysis, and enzymatic treatment to ensure efficient protein release.
The extracted proteins are then typically separated by two-dimensional gel electrophoresis (2-DE) or liquid chromatography. The major challenge at this stage lies in handling the high complexity and wide dynamic range of microbial samples, as well as effectively removing contaminants that may interfere with downstream mass spectrometry and bioinformatics analyses.
LC-MS/MS Analysis and Peptide Identification
Proteomic methodologies can generally be divided into two categories: shotgun (bottom-up) proteomics and top-down proteomics. Shotgun proteomics, the more widely adopted method, digests proteins into peptides before analysis by mass spectrometry. It is robust and efficient for protein identification and quantification. Top-down proteomics analyzes intact proteins, providing insights into sequence, structure, and modifications, but is technically demanding and rarely applied to metaproteomics.
In a typical bottom-up workflow, proteins are digested—most often with trypsin—into peptides. These are separated by liquid chromatography and analyzed with tandem mass spectrometry (LC-MS/MS). Peptides are ionized and detected as MS1 spectra, then fragmented to produce MS2 spectra. These spectra are interpreted by proteomics software and matched to theoretical spectra from protein databases. This approach enables comprehensive identification and quantification of peptides and their corresponding proteins.
Database Search and Functional Annotation
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) forms the core of modern metaproteomics. High-resolution mass spectrometers can detect thousands of proteins within complex mixtures. Large-scale protein identification typically uses the shotgun proteomics strategy, with database searches as the key step.
In microbiome studies, customized databases derived from metagenomic or metatranscriptomic datasets are often required to improve accuracy and coverage. Bioinformatics tools such as MaxQuant and Proteome Discoverer are used to align mass spectrometry data with reference sequences. Identified proteins are then annotated using resources like GO and KEGG, revealing key metabolic pathways and biological processes.
Applications of Metaproteomics in Microbiome Research
Metaproteomics has extensive applications across microbiome research. In environmental microbiology, it helps elucidate how microbes respond to environmental changes and pollutants. In industrial biotechnology, it supports the optimization of microbial fermentation processes and biofuel production.
In the broader field of microbiome studies, metaproteomics is applied to human microbiomes (oral, skin, gut, lung, vaginal), animal microbiomes (farm, wild, laboratory animals), environmental microbiomes (soil, marine), and even ancient microbiome samples. Among these, human microbiome research represents one of the most significant areas of application. By analyzing microbial protein expression in body sites such as the gut, mouth, and skin, researchers have uncovered profound links between microbes and human health.
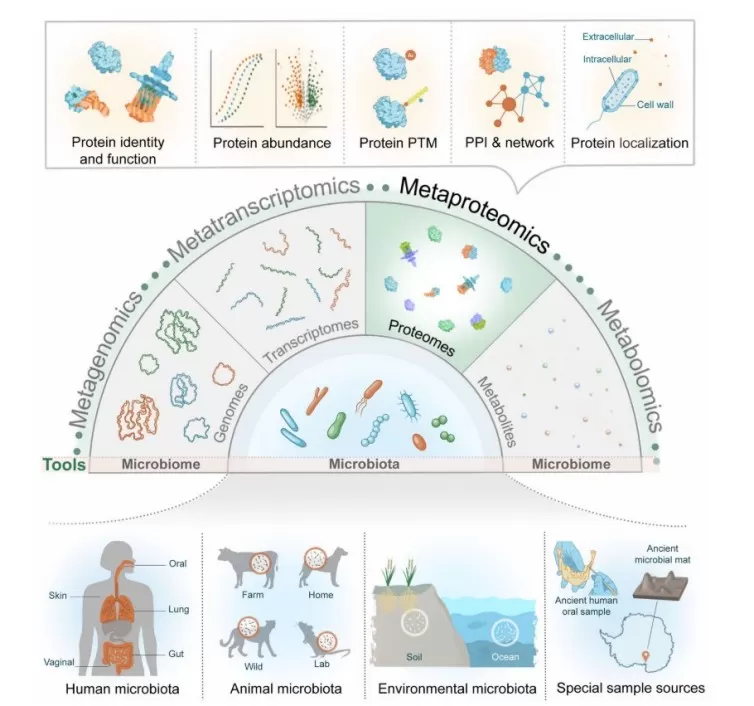
Applications of Metaproteomics in Microbiome Research
Image source: Van Den Bossche T., et al. (2025). Imeta, May 6; 4(3):e70031. doi: 10.1002/imt2.70031.
Gut Microbiota, Host Interaction, and Diet: Insights from Metaproteomics
The intricate interactions among the gut microbiota, host, and diet are fundamental to maintaining health or driving disease. Traditional methods such as metagenomic sequencing primarily reveal microbial genetic composition but cannot capture functional activity. As the direct executors of cellular processes, proteins provide a more accurate reflection of the dynamic functional states of both microbes and hosts. Consequently, gut microbiota–host interactions are a central focus of metaproteomics.
In February 2025, Cell published a landmark study led by Eran Elinav at the Weizmann Institute of Science. The team developed a metaproteomic approach based on metagenome-informed databases (MIM, Metagenome-informed Metaproteomics). For the first time, they performed analyses using integrated databases that combined microbial metagenomes, host (human or murine) proteomes, and nearly 300 dietary proteomes. This enabled cross-scale integration of microbial functions, host responses, and dietary exposures. The study addressed key limitations of conventional metagenomics, delivering a species-level functional resolution tool with strong translational potential in complex conditions such as inflammatory bowel disease (IBD).
Metaproteomics captures the “protein tools” actively produced by bacteria, revealing specific functional shifts in microbial communities. Whereas traditional omics approaches speculate on microbial potential, metaproteomics pinpoints actual activities, providing a solid foundation for precision nutrition rather than conjecture.
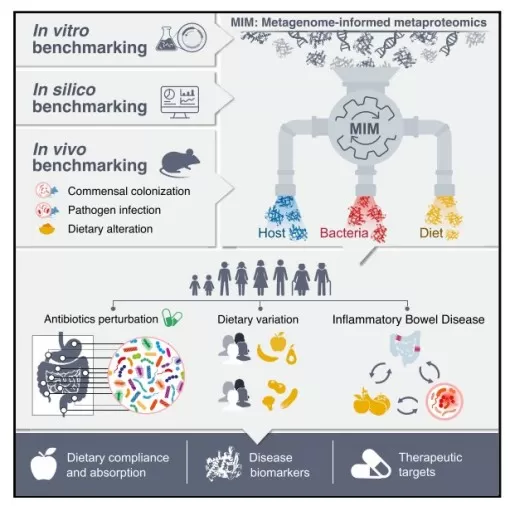
Overview of Experimental Workflow
Image source: Valdés-Mas R., et al. (2025). Cell, 188(4):1062-1083.e36. doi:10.1016/j.cell.2024.12.016.
In October 2024, Maria T. Abreu and colleagues at the University of Miami Miller School of Medicine published a study in Microbiome titled “Metaproteomics reveals diet-induced changes in gut microbiome function according to Crohn’s disease location.” Combining metaproteomics with PRM-targeted quantitative proteomics, they found that patients with ileal Crohn’s disease exhibited a pronounced loss of carbohydrate metabolism functions, particularly enzymes associated with Faecalibacterium. In contrast, colonic Crohn’s patients showed stronger responses to dietary interventions. This finding explains why identical diets produce markedly different therapeutic outcomes depending on disease location—highlighting the need for tailored nutritional strategies in ileal disease.
By analyzing gut microbial protein expression profiles, researchers not only advanced understanding of host–microbiota interactions but also identified novel therapeutic targets for the development of new treatments.
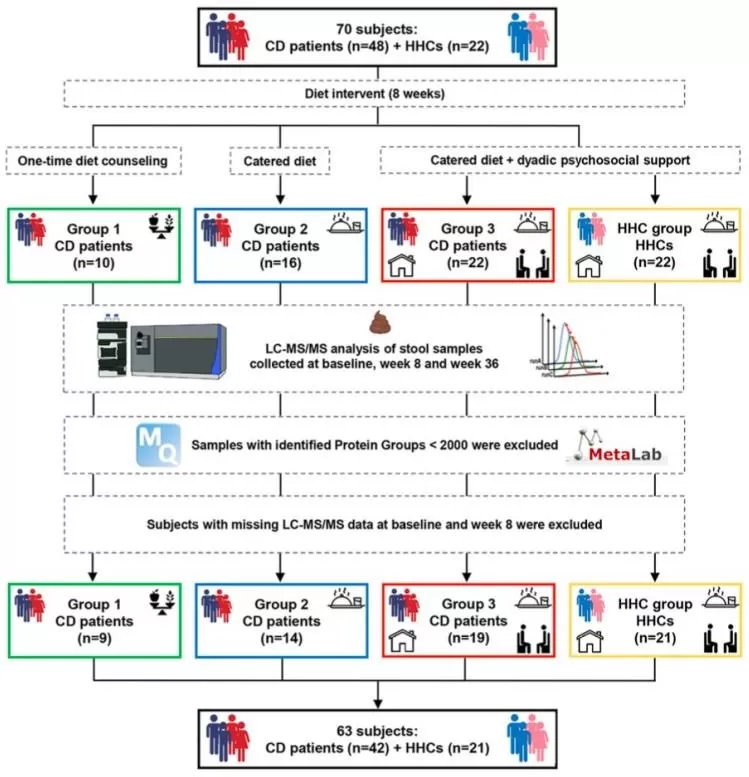
Participant Sample Size and Experimental Workflow
Image source: Mortera L. S., et al. (2024). Microbiome, 12(1):217-217. doi: 10.1186/s40168-024-01927-5.
Challenges and Future Trends in Metaproteomics
Despite rapid advances, metaproteomics continues to face significant challenges. These include difficulties in protein identification due to sample complexity, incomplete databases, and the limitations of current data analysis algorithms.
Future directions involve improving the sensitivity of mass spectrometry, developing more efficient methods for data integration, and establishing standardized analytical workflows. Progress in single-cell proteomics is also expected to propel metaproteomics toward higher-resolution analyses.
Another major trend is the integration of metaproteomics with other omics approaches—metagenomics, metatranscriptomics, and metabolomics—to provide a more comprehensive and detailed understanding of microbial community functions.
Summary: Why Metaproteomics Matters in Multi-Omics
Metaproteomics plays an indispensable role in multi-omics research. As the key bridge connecting genomic potential with phenotypic function, it directly reflects the true functional state of microbial communities. Compared with other omics technologies, metaproteomics provides the closest approximation to functional phenotypes, revealing what microbes are actually doing.
Within systems biology, the integration of metaproteomics with metagenomics, metatranscriptomics, and metabolomics enables the construction of comprehensive functional maps of microbial communities. This integrated approach is transforming our understanding of complex microbial ecosystems and shows great promise in fields such as precision medicine, environmental science, and industrial biotechnology.
With ongoing technological advances and decreasing costs, metaproteomics is poised to become a routine tool in microbiome research, offering unprecedented functional insights into the microscopic world of life.
References
1. Van Den Bossche T, Armengaud J, Benndorf D, et al. The microbiologist's guide to metaproteomics. Imeta. 2025 May 6;4(3):e70031. doi: 10.1002/imt2.70031.
2. Valdés-Mas R, Leshem A, Zheng D, et al. Metagenome-informed metaproteomics of the human gut microbiome, host, and dietary exposome uncovers signatures of health and inflammatory bowel disease. Cell. 2025;188(4):1062-1083.e36. doi:10.1016/j.cell.2024.12.016.
3. Mortera L S, Marzano V, Rapisarda F, et al. Metaproteomics reveals diet-induced changes in gut microbiome function according to Crohn’s disease location. Microbiome. 2024;12(1):217-217. doi: 10.1186/s40168-024-01927-5.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


