Proteoforms in Proteomics: Definition, Biological Importance, and Detection Technologies
What Are Proteoforms and Why They Matter in Modern Proteomics
Proteoforms are structurally or functionally distinct variants of a protein that originate from the same gene through mechanisms such as alternative splicing and post-translational modifications (PTMs).
If the genome represents the static blueprint of life, then the proteome is its dynamic manifestation — the “construction site” that reveals biological activities in real time. The complexity of the proteome far exceeds that of the genome. A single gene can produce multiple mRNA transcripts via alternative splicing, which are then translated into various protein isoforms. In addition, proteins often undergo numerous chemical modifications after translation, such as phosphorylation, glycosylation, and acetylation. These post-translational modifications greatly expand protein functional diversity. Although the human genome contains roughly 20,000 genes, it is estimated to encode more than one million distinct proteoforms.
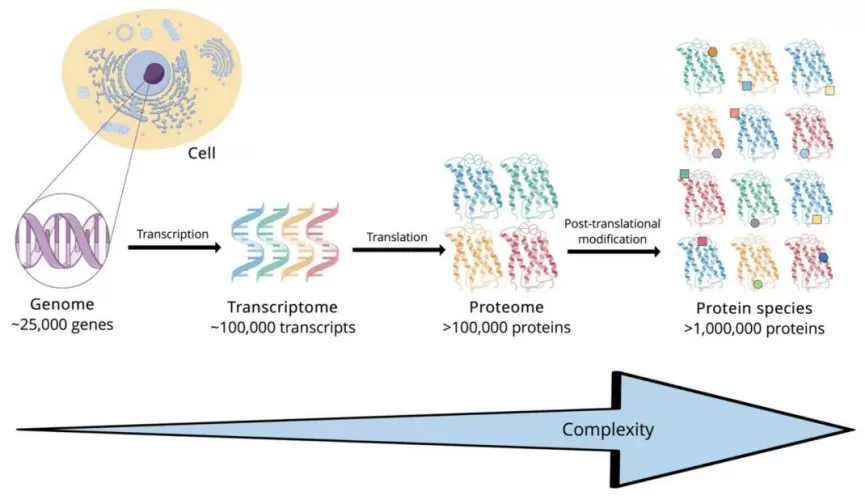
The complexity from genome to proteome
These variants typically share a similar core structure and function, yet some display unique biochemical or physiological characteristics. Understanding these subtle differences is essential for uncovering how proteins regulate cellular behavior and disease processes.
Core Cellular Functions of Proteins
Proteins serve as the molecular workforce of cells, driving nearly every biological process with remarkable diversity and specificity.
Structural Support: Proteins act as the architectural framework of cells and tissues. For example, collagen strengthens connective tissues, keratin forms hair and nails, and cytoskeletal proteins maintain cellular shape and integrity.
Catalytic Activity: Most enzymes are proteins functioning as highly efficient biological catalysts. They accelerate cellular chemical reactions with exquisite precision and control.
Transport and Storage: Many proteins act as molecular carriers. Hemoglobin transports oxygen in the bloodstream, while membrane transporters mediate the movement of molecules across cellular membranes.
Signal Transduction and Regulation: Proteins are crucial for cellular communication. Hormones like insulin serve as signaling molecules that regulate metabolism, while membrane receptors detect and transmit external cues.
Immune Defense: Antibodies are specialized immune proteins capable of specifically binding to invading pathogens to trigger immune responses.
Movement and Motility: Muscle contraction depends on the coordinated action of actin and myosin, and intracellular cargo transport is driven by motor proteins moving along cytoskeletal filaments.
Sources of Proteoform Diversity
The enormous diversity of proteoforms arises primarily from three biological mechanisms:
Genetic Variation
Genetic variation refers to changes in genes or chromosomal structures, including point mutations, insertions, or deletions. Such variations can alter the amino acid sequence or functional properties of proteins, generating diverse proteoforms. In crop breeding, genetic variation is utilized to improve yield or disease resistance through gene editing. In medical research, point mutations in specific genes are linked to hereditary diseases by disrupting normal protein function.
Alternative Splicing
Alternative splicing allows a single pre-mRNA transcript to be processed in multiple ways, combining exons in various configurations. This produces mRNA isoforms that translate into structurally and functionally distinct proteins. It is one of the major contributors to proteomic complexity in eukaryotic organisms.
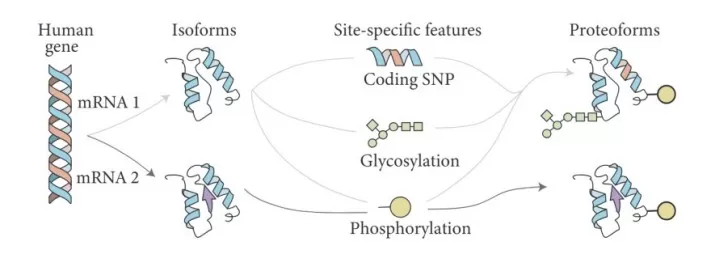
Alternative splicing produces different proteoforms
Alternative Promoter Usage
Different transcription start sites (promoters) can lead to structurally distinct transcripts.
For example, in cotton, alternative splicing of the GhLSM1B gene was shown to enhance callus proliferation and influence somatic embryogenesis, providing a new strategy to improve transformation efficiency.
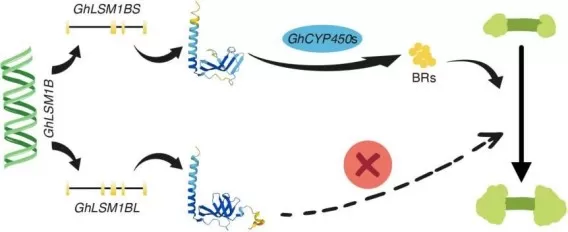
GhLSM1B splicing regulates cotton somatic embryogenesis through CYP450-mediated BR synthesis. (image source: Yang Z, He J. et al., Plant Biotechnol J. 2025 Jul;23(7):2670–2672)
In cancer biology, abnormal RNA splicing generates novel protein isoforms that can promote tumor progression. A recent full-length transcriptomic atlas of gallbladder cancer revealed that alternative splicing of the ERBB2 gene drives tumor development and confers resistance to trastuzumab.
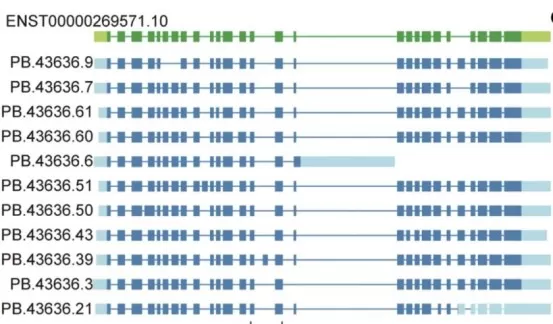
Structure of eleven selected ERBB2 novel isoforms. (image source: Wang Z. et al., Signal Transduct Target Ther 10, 54 (2025))
Post-Translational Modifications: Key Drivers of Proteoform Diversity
Post-translational modifications (PTMs) are chemical changes that occur after translation, such as phosphorylation, glycosylation, and acetylation. PTMs can alter protein structure, stability, and activity, giving rise to numerous proteoforms with distinct biological roles.
In plant research, PTMs have been found to regulate responses to environmental stresses—for instance, glycosylation enhances protein stability and stress tolerance.
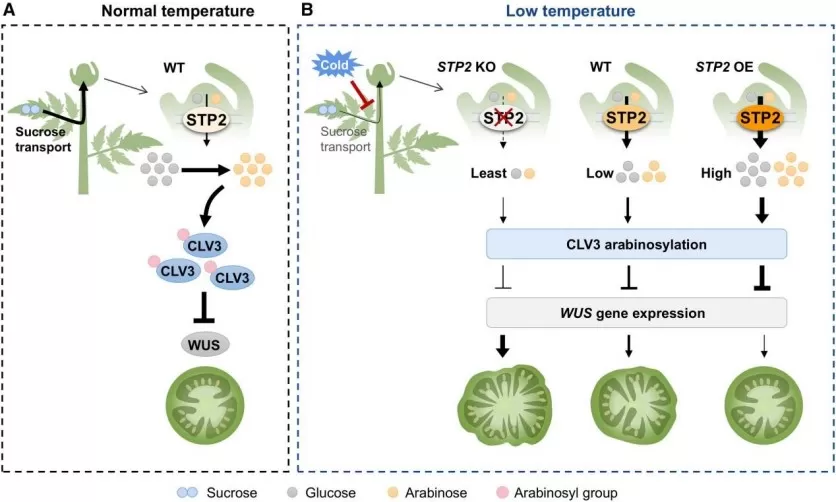
STP2-Mediated Sugar Transport Regulates CLV3 Arabinosylation and Maintains Tomato Fruit Locule Development under Low-Temperature Stress. (image source: Li Y, Wang J. et al., Mol Plant. 2025;18(6):1014–1028)
In biomedical studies, PTMs play crucial roles in modulating enzyme activity and signaling pathways. Phosphorylation, for example, can activate or inhibit enzymes, influencing disease onset and progression.
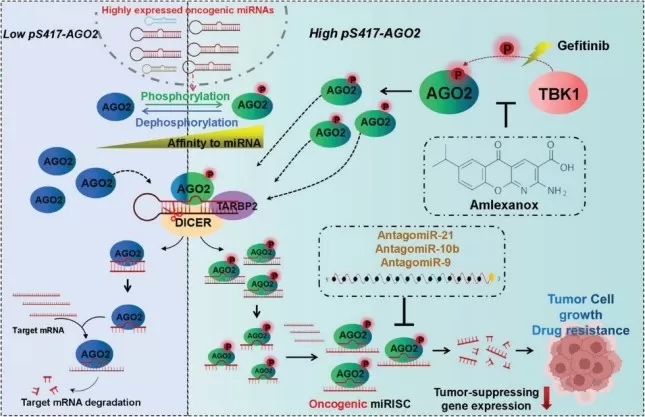
TBK1-mediated pS417-AGO2 promotes NSCLC progression and resistance to Gefitinib by increasing the formation and activity of oncogenic miRISCs. (image resource: Zhao X, Cao Y. et al., Adv Sci (Weinh). 2024;11(15):e2305541)
Proteoform Detection Technologies in Modern Proteomics
Classical Biochemical and Separation Techniques
Before the rise of high-throughput proteomics, researchers used traditional biochemical approaches to separate and identify protein variants based on their physicochemical properties. Though limited in sensitivity, these techniques remain valuable for specific applications.
Electrophoresis:
A time-tested method for separating proteins. Isoelectric focusing (IEF) differentiates variants by their isoelectric points, while polyacrylamide gel electrophoresis (PAGE) separates proteins by molecular weight or charge. These techniques are still used for the preliminary screening of protein variants such as hemoglobin isoforms.
High-Performance Liquid Chromatography (HPLC):
HPLC, including ion-exchange and reverse-phase chromatography, provides high resolution, sensitivity, and automation. Clinically, HPLC remains the gold standard for detecting hemoglobin variants in conditions such as thalassemia.
Spectroscopic and Physical Analyses:
Techniques such as circular dichroism (CD) and Fourier-transform infrared spectroscopy (FTIR) detect conformational changes caused by amino acid substitutions. Differential scanning calorimetry (DSC) evaluates the impact of variants on protein thermal stability.
While reliable, these classical methods are inherently low-throughput and cannot meet the depth and scale demanded by precision proteomics.
Mass Spectrometry–Based Proteomics
Mass spectrometry (MS) serves as the analytical backbone of modern proteomics, offering unmatched sensitivity, accuracy, and throughput for proteoform detection.
Data-Dependent Acquisition (DDA):
Also known as shotgun proteomics, DDA performs an initial MS1 scan of all peptide ions, then selectively fragments the most abundant ions (MS2) for sequencing. DDA is ideal for protein discovery but less effective for detecting low-abundance peptides due to its stochastic sampling.
Data-Independent Acquisition (DIA, e.g., SWATH-MS):
DIA addresses DDA’s limitations by systematically fragmenting all ions across consecutive mass windows, generating a comprehensive digital fragment ion map. By matching this data against spectral libraries, DIA enables reproducible and quantitative profiling of thousands of proteins. Owing to its data completeness, SWATH-MS is particularly suitable for large-scale clinical cohort studies.
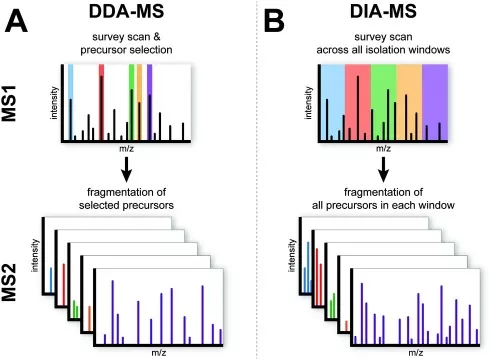
Mechanism of DIA and DDA
Targeted Proteomics (MRM/PRM):
When studying specific known proteins, Multiple Reaction Monitoring (MRM) and Parallel Reaction Monitoring (PRM) deliver the highest sensitivity and quantitative precision. These targeted assays selectively monitor specific peptide transitions, enabling reliable quantification of low-abundance proteoforms in complex biological samples such as plasma. They are often regarded as the gold standard for quantitative proteomics.
Ultra-Sensitive Immunoassay Platforms for Proteoform Detection
For clinical specimens where protein concentrations are extremely low (in the pg/mL to fg/mL range), conventional ELISA or standard MS techniques often lack sufficient sensitivity. Emerging ultra-sensitive immunoassay platforms such as Olink and Simoa have filled this technological gap.
Olink Proteomics Platform:
The Proximity Extension Assay (PEA) used by Olink employs paired antibodies conjugated with DNA oligonucleotides that bind distinct epitopes on the target protein. When both antibodies are in close proximity, their DNA tags hybridize and extend, forming a unique DNA reporter molecule detectable by qPCR or next-generation sequencing (NGS). This dual-recognition system ensures high specificity and minimizes cross-reactivity. The Olink platform combines high throughput, ultra-high sensitivity (fg/mL), and minimal sample requirements (1 μL of plasma), making it ideal for biomarker discovery and clinical validation.
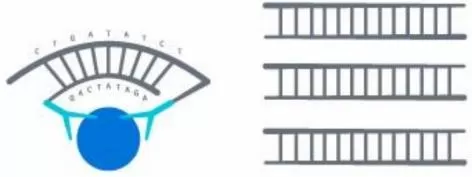
The Proximity Extension Assay
Simoa (Single-Molecule Array) Platform:
Simoa pushes immunoassay sensitivity to the single-molecule level. Millions of antibody-coated microbeads are arrayed in femtoliter-sized wells, each containing at most one target molecule. After binding and enzymatic amplification, fluorescent wells are digitally counted, allowing absolute quantification. Simoa achieves sensitivity over 1,000 times higher than ELISA, with detection limits reaching fg/mL or even attogram levels, making it invaluable for neurodegenerative disease and cancer biomarker monitoring.
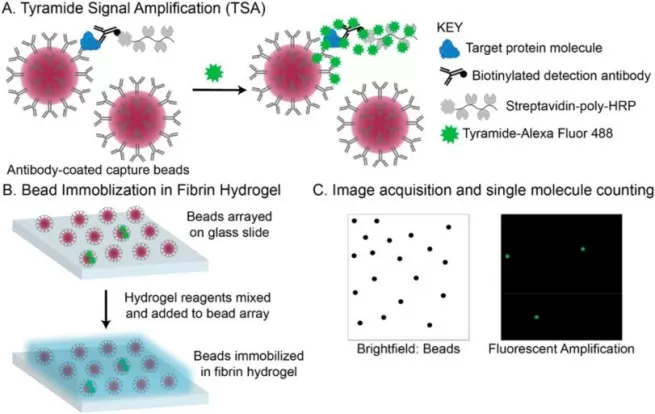
Workflow of Single-Molecule Array (image source: Dong R. et al., Talanta. 2024;270:125529)
Reference:
1. Yang Z, He J, Yao S, et al. Identification and overexpression of RNA-decapping protein GhLSM1BS: Enhancing cotton somatic embryogenesis through up-regulating brassinosteroid biosynthesis. Plant Biotechnol J. 2025;23(7):2670-2672. doi:10.1111/pbi.70090
2. Wang Z, Gao L, Jia Z, et al. Full-length transcriptome atlas of gallbladder cancer reveals trastuzumab resistance conferred by ERBB2 alternative splicing. Signal Transduct Target Ther. 2025;10(1):54. Published 2025 Feb 14. doi:10.1038/s41392-025-02150-w
3. Li Y, Wang J, Liang X, et al. STP2-mediated sugar transport in tomato shoot apices is critical for CLV3 arabinosylation and fruit locule development under low temperatures. Mol Plant. 2025;18(6):1014-1028. doi:10.1016/j.molp.2025.05.002
4. Zhao X, Cao Y, Lu R, et al. Phosphorylation of AGO2 by TBK1 Promotes the Formation of Oncogenic miRISC in NSCLC. Adv Sci (Weinh). 2024;11(15):e2305541. doi:10.1002/advs.202305541
5. Dong R, Yi N, Jiang D. Advances in single molecule arrays (SIMOA) for ultra-sensitive detection of biomolecules. Talanta. 2024;270:125529. doi:10.1016/j.talanta.2023.125529
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


