Sphingosine: What It Is, Biosynthesis, and Roles in Health & Disease
Sphingosine may not be a household name, but this lipid metabolite plays critical roles in our bodies – from building skin barriers to balancing life-and-death signals inside cells. Understanding sphingosine opens a window into how our cells fight infections, regulate inflammation, and even how new therapies (like certain multiple sclerosis drugs) work. In this blog, we’ll explore what sphingosine is, how it’s made and metabolized, its difference from related lipids, and why it matters for human health, plant biology, and everyday life. By the end, you’ll see why sphingosine, though enigmatic, has earned its name inspired by the Sphinx and why researchers are so interested in this “riddle” of a molecule.
What is Sphingosine? Structure and Properties
Sphingosine is defined as the major naturally occurring long-chain base found in sphingolipids. Chemically, it is an (unsaturated) C18 amino alcohol with two hydroxyl groups and one amine group (often denoted as d18:1 for its 18-carbon sphingoid backbone with one double bond). This unique structure (commonly the D-erythro configuration in mammals) enables sphingosine to serve as the backbone for complex sphingolipids: when a fatty acid is attached to sphingosine’s amine group, it forms ceramide, and further addition of headgroups (like phosphocholine in sphingomyelin or sugars in glycosphingolipids) generates the diverse sphingolipid family. In pure form, sphingosine is a lipid that is hydrophobic yet slightly amphiphilic due to its polar groups, which means it localizes to cellular membranes (especially the cytosolic leaflet) and lipid droplets.
Unveiling Sphingosine: Discovery and Structure Story
The story of sphingosine’s discovery dates back to the 19th century. In 1884, Johann L. W. Thudichum, a German physician-chemist studying brain tissues, isolated an enigmatic lipid component. Finding its behavior puzzling, he named it “sphingosine” after the Sphinx. For decades, its exact structure remained a riddle. It wasn’t until 1947 that biochemist Herbert Carter and colleagues finally elucidated sphingosine’s structure as an unsaturated C18 amino diol. We now know sphingosine’s formula is C18H37NO2, and structurally it is the “long-chain base” of sphingolipids. Sphingosine’s structure includes a trans double bond (making it an alkene), an amino group at carbon-2, and hydroxyls at carbons-1 and -3. This unique configuration allows sphingosine to link with fatty acids and headgroups to form more complex sphingolipids. Notably, free sphingosine in cells is usually very low, because it’s quickly utilized or stored in other forms. It primarily exists as part of ceramides (where a fatty acid is amide-linked to the amino group) and sphingomyelin (ceramide plus a phosphocholine head) in cell membranes. The historical fascination with sphingosine has only grown as we recognize it not just as a structural lipid, but also as a critical signaling molecule.
Sphingosine vs. Sphinganine vs. Ceramide vs. S1P: The Sphingolipid Rheostat
Sphinganine (d18:0; dihydrosphingosine) is the saturated version of sphingosine and a key intermediate in sphingolipid biosynthesis. Ceramide is not sphingosine itself but a conjugate in which sphingosine (or sphinganine) carries a fatty acid via an amide bond; adding a phosphocholine headgroup to ceramide yields sphingomyelin, a major membrane lipid. These molecules form the “sphingolipid rheostat,” a signaling balance that steers cell fate. Generally, ceramide and sphingosine favor apoptosis, while sphingosine-1-phosphate (S1P)—the phosphorylated, diffusible form—drives survival, proliferation, and migration. Enzymes tighten the interconversion: ceramidases release sphingosine from ceramide; sphingosine kinases (SPHK1/2) generate S1P; S1P phosphatases regenerate sphingosine; ceramide synthases (CerS) re-acylate sphingosine in the salvage pathway; and S1P lyase irreversibly degrades S1P, removing it from the cycle. In short, sphinganine and sphingosine are the base backbones (differing by saturation), ceramide is the storage/structural acylated form, and S1P is the pro-survival signal. Shifts in this rheostat—toward ceramide/sphingosine or toward S1P—profoundly influence cell death versus survival programs.
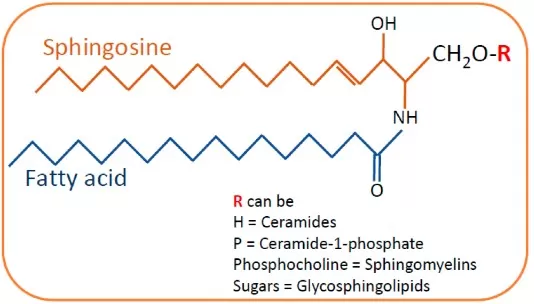
General Structure of Sphingolipids: Sphingosine, Sphinganine, and Ceramide Relationships
Source: Mallela SK, Merscher S, Fornoni A. “Implications of Sphingolipid Metabolites in Kidney Diseases,” International Journal of Molecular Sciences (2022) 23(8):4244. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
How Is Sphingosine Made? – Biosynthesis Pathway
De novo sphingolipid biosynthesis starts in the endoplasmic reticulum when serine palmitoyltransferase (SPT) condenses L-serine with palmitoyl-CoA to form 3-ketodihydrosphingosine. 3-KDSR then reduces this intermediate to sphinganine (dihydrosphingosine; d18:0). Next, ceramide synthases (CerS1–6) N-acylate sphinganine with fatty acyl-CoAs of defined chain length to produce dihydroceramide, which dihydroceramide desaturase (DEGS/Des1) converts to ceramide by introducing the 4,5-trans double bond. Functionally, ceramide is the hub for complex sphingolipids: sphingomyelin (via sphingomyelin synthase), glycosphingolipids (via UGCG and downstream glycosyltransferases), and ceramide-1-phosphate. Free sphingosine (d18:1) is largely generated by ceramidases in the salvage pathway, not directly by the de novo route; it can be re-acylated by CerS or phosphorylated by sphingosine kinases (SphK1/2) to S1P. Off-pathway, SPT can use alanine or glycine to form deoxysphingolipids that resist normal catabolism and may be cytotoxic. In short, the ER-based de novo pathway builds sphinganine → (CerS) dihydroceramide → (DEGS) ceramide, while salvage reactions supply free sphingosine and interconvert species that feed signaling (S1P) and membrane-building branches—key checkpoints for cell fate and lipid homeostasis.
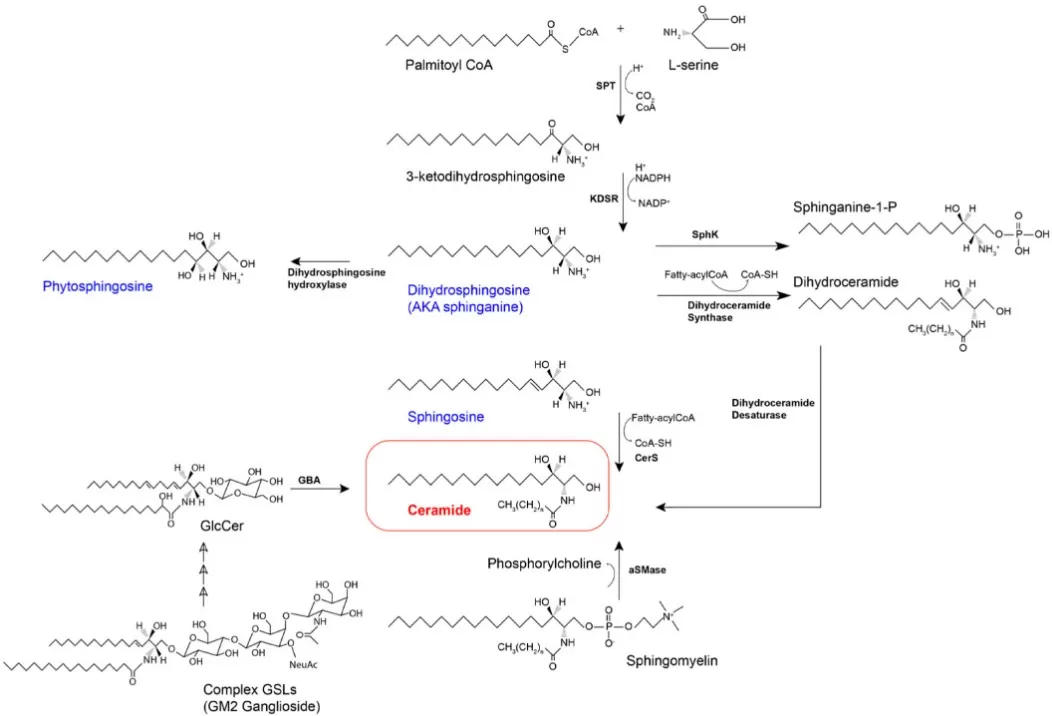
De novo Sphingolipid Biosynthesis and Salvage Pathways Leading to Ceramide and Sphingosine
Source: Quinville BM, Deschenes NM, Ryckman AE, Walia JS. “A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis,” International Journal of Molecular Sciences (2021) 22(11):5793. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
How Is Sphingosine Metabolized in the Body?
Sphingosine is a short-lived, bioactive sphingoid base that cells rapidly channel into three main routes. First, sphingosine kinases SPHK1/2 phosphorylate it at C1 to generate sphingosine-1-phosphate (S1P), a potent signaling lipid. S1P can be dephosphorylated by S1P phosphatases (SGPP1/2) to regenerate sphingosine, or irreversibly cleaved by S1P lyase (SGPL1) to ethanolamine phosphate and hexadecenal—an exit from sphingolipid metabolism that feeds glycerophospholipid and fatty-acid pathways. Second, sphingosine is re-acylated by ceramide synthases (CerS1–6) to form ceramide in the salvage pathway, keeping free sphingosine low and replenishing membrane and signaling pools. Notably, some ceramidases can operate near-reverse under cellular conditions, further tuning the sphingosine↔ceramide balance. Third, lysosomal turnover of complex sphingolipids (e.g., sphingomyelin, glycosphingolipids) produces ceramide, which acid ceramidase hydrolyzes to sphingosine that is exported to the cytosol for reuse. Together, phosphorylation/dephosphorylation, acylation/deacylation, and lyase-mediated breakdown create a tightly regulated rheostat linking sphingosine, S1P, and ceramide—so that small shifts in enzyme activity translate into large changes in cell survival, migration, inflammation, and stress responses.
Sphingosine isn’t just a passive building block; it actively influences health and disease. Recent research reveals its involvement in everything from immune defense to cancer progression. Below, we introduce a few key areas – each highlighting how sphingosine or its related enzymes impact human biology.
Skin Barrier Defender: Sphingosine as a Natural Antimicrobial Shield
One of sphingosine’s remarkable roles is serving as an innate antimicrobial agent on our skin and mucous membranes. Our epidermis (especially the stratum corneum) contains free sphingosine as part of the skin lipid mixture, where it helps keep microbial invaders in check. Studies have shown that sphingosine at physiologic levels can kill bacteria such as Staphylococcus aureus, a common skin pathogen. In fact, conditions like atopic dermatitis, where skin infections are common, are associated with significantly lower levels of sphingosine in the skin. Without enough sphingosine, the skin becomes more hospitable to Staph and other germs. Mechanistically, sphingosine can insert into bacterial membranes (it’s a cationic lipid) and disrupt them, leading to bactericidal activity. This has inspired research into therapeutic uses – for example, inhaled sphingosine is being explored in cystic fibrosis models to sterilize airways; it restored sphingosine levels in CF mice and protected them from Pseudomonas aeruginosa lung infection. While directly applying sphingosine as an antibiotic is still experimental, its potent microbe-killing ability and presence in skin creams (often as phytosphingosine in acne treatments) highlight its value. Nature evolved sphingosine as a “chemical shield” on our epithelial surfaces, underscoring the link between lipid metabolism and immune defense.
Balancing Life and Death: Sphingosine in Cancer Signaling
The sphingolipid rheostat is central to cancer biology. A high ceramide/sphingosine-to-S1P ratio drives pro-apoptotic signaling, whereas shifting toward S1P favors survival, proliferation, and migration. Many tumors exploit this by upregulating sphingosine kinase 1 (SPHK1) to boost S1P and limit ceramide buildup, helping them evade apoptosis. Experimentally, exogenous sphingosine or ceramide can trigger cell-cycle arrest and programmed cell death; sphingosine activates protein phosphatases, inhibits kinases such as PKC, and at higher levels engages caspase-dependent apoptosis. Conversely, excess S1P from hyperactive SPHK1 promotes angiogenesis and metastasis via S1P receptors (S1PR1–3) on tumor and endothelial cells. These observations underpin strategies to “tilt” the rheostat in oncology: SPHK inhibitors, S1P receptor modulators, and ceramide analogs aim to restore pro-death signaling in cancer cells. In short, sphingosine functions as a brake on tumor survival—when its levels rise (or S1P falls), malignant cells are more likely to undergo apoptosis. The therapeutic challenge is to rebalance this lipid network to selectively kill cancer cells while sparing normal tissues.
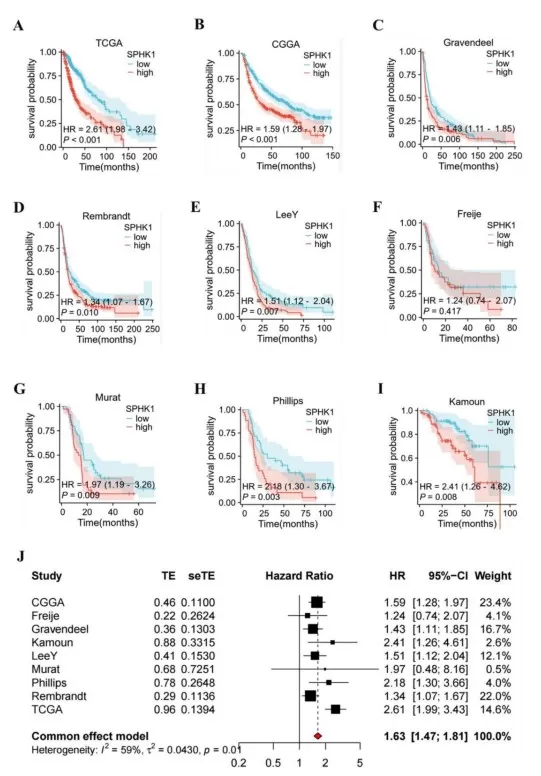
High SPHK1 Expression Predicts Worse Overall Survival in Glioma (Kaplan–Meier and Meta-analysis)
Source: Song Z, Zhao Z, Liu X, et al. “Sphingosine kinase 1 promotes M2 macrophage infiltration and enhances glioma cell migration via the JAK2/STAT3 pathway,” Scientific Reports (2025) 15:4152. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Nerve Signals and Neuroinflammation: Sphingosine’s Role in the Brain
Sphingosine and its metabolites orchestrate neural signaling and neuroimmune balance. In the CNS, free sphingosine and S1P act beyond structural sphingolipids: S1P generated by SPHK1/2 modulates astrocyte and microglia activation, neural progenitor migration, and blood–brain barrier integrity. In neuroinflammation, isoform context matters. Cytosolic SPHK1 generally amplifies inflammatory signaling and leukocyte recruitment via S1P–S1PR1/3. By contrast, nuclear SPHK2 can produce S1P that inhibits HDAC1/2, reshapes gene expression, and biases microglia toward an anti-inflammatory, tissue-repairing M2 state—consistent with in vivo data where the SphK2-dependent phosphorylation of fingolimod (FTY720) reduces IL-1β/TNF-α and increases TREM2+/Iba1+ microglia after ischemic injury. Subcellular localization further tunes outcomes: nuclear S1P regulates epigenetic programs, while extracellular S1P signals through S1P receptors to control immune-cell egress (mechanism of fingolimod in MS). Disrupted sphingosine handling also links to neurodegeneration (e.g., lysosomal sphingosine buildup in Niemann-Pick type C perturbs calcium homeostasis). Overall, sphingosine/S1P signaling is context-dependent—therapies targeting SPHK isoforms or S1P receptors aim to quell neuroinflammation while preserving neural repair.
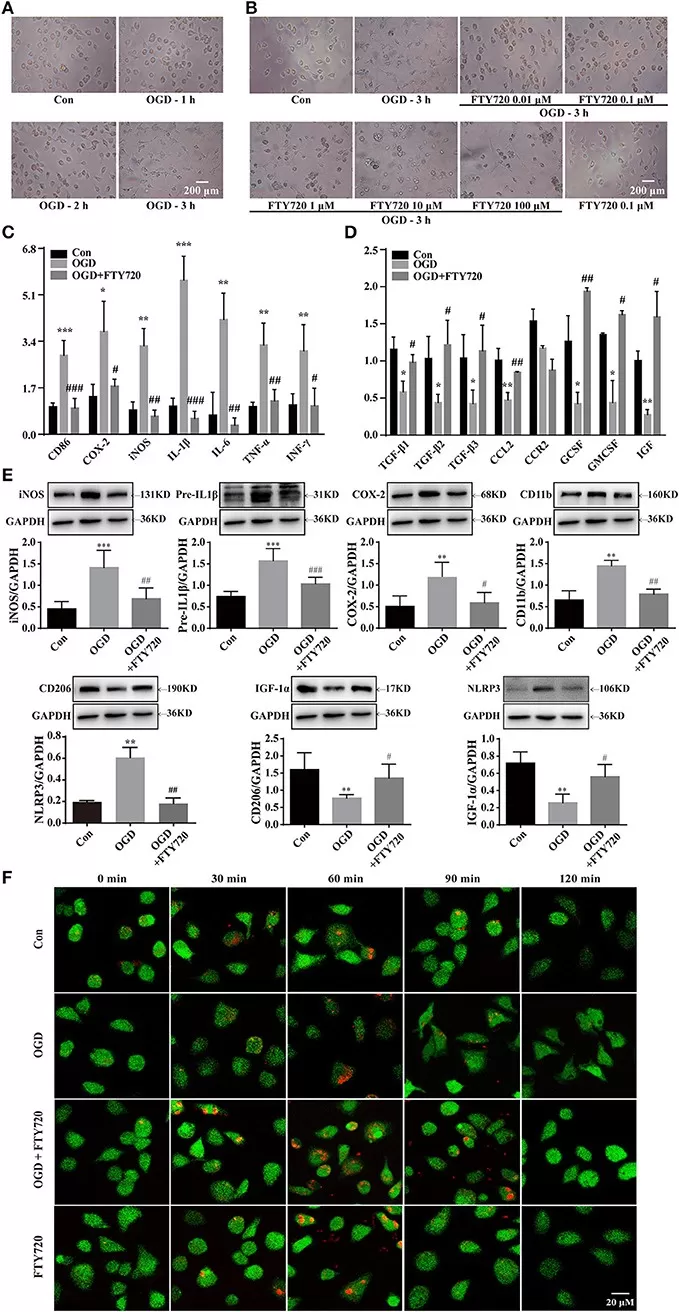
SphK2-dependent Fingolimod Phosphorylation Drives Microglia Toward an M2 Anti-inflammatory Phenotype After Stroke
Source: Ji J, Wang J, Yang J, et al. “The Intra-nuclear SphK2-S1P Axis Facilitates M1-to-M2 Shift of Microglia via Suppressing HDAC1-Mediated KLF4 Deacetylation,” Frontiers in Immunology (2019) 10:1241. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
Plant Sphingolipids: Phytosphingosine, S1P Signaling, and Stress Defense
Plants predominantly use phytosphingosine (t18:0) as their sphingoid base, with smaller amounts of Δ4-unsaturated bases. Beyond membrane structure (rich glycosylated sphingolipids stabilizing the plasma membrane), plant sphingolipids act as potent signals in stress responses. In drought, abscisic acid (ABA) boosts sphingosine-1-phosphate (S1P) production in guard cells; S1P then drives stomatal closure, conserving water. Arabidopsis mutants lacking sphingosine kinase (SPHK) or S1P signaling show impaired closure, underscoring S1P’s role in ABA–S1P guard-cell signaling. During pathogen attack, specific ceramide species and long-chain bases accumulate to trigger programmed cell death (PCD) at infection sites—the hypersensitive response that blocks biotrophic pathogens and enhances resistance. Disrupting sphingolipid metabolism (e.g., with fumonisins, which inhibit ceramide synthesis) causes growth defects and stress hypersensitivity, highlighting their developmental importance. In short, plant sphingolipids integrate membrane integrity with signal transduction: phytosphingosine provides the scaffold; S1P mediates drought tolerance via guard cells; and ceramide-driven PCD fortifies immune defense. The parallels with animal systems reveal a conserved rheostat logic—using sphingolipid cues to balance survival, growth, and defense under environmental stress.
Sphingosine in Daily Life: Medicine, Diet, and Skincare
You won’t see “sphingosine” on labels, but its biology touches everyday life. Dietary sphingolipids in dairy and soy are digested to ceramide and sphingosine, metabolites studied for supporting colon health by promoting controlled apoptosis in abnormal cells. In medicine, the standout is fingolimod (FTY720)—a sphingosine analog phosphorylated in vivo to an S1P receptor modulator that sequesters lymphocytes, delivering effective oral therapy for multiple sclerosis. In skincare, ceramide-rich creams rebuild the skin barrier in eczema, while phytosphingosine (a natural skin lipid) is used for its antimicrobial and anti-inflammatory actions in acne- and rosacea-prone skin. Emerging ideas include inhaled sphingosine to help reduce airway infections (e.g., ventilator-associated pneumonia or cystic fibrosis)—an active research area rather than standard care. Meanwhile, oncology and diagnostics are exploring SPHK inhibitors and lipidomic biomarkers (circulating sphingolipids) for precision health. The thread across these uses is simple: by leveraging sphingosine’s roles in cell signaling and barrier integrity, products and therapies translate complex lipid biology into tangible benefits.
Measuring Sphingosine: Concise Methods
Targeted LC–MS/MS
Samples are extracted with organic solvents and quantified by LC–MS/MS using isotope-labeled internal standards (e.g., d7-sphingosine) in MRM mode. Delivers high specificity/sensitivity and absolute concentrations of sphingosine, sphinganine, S1P, and ceramides—best for biomarkers and clinical studies.
Untargeted UHPLC–HRMS
High-resolution MS (Orbitrap/TOF) profiles hundreds of sphingolipids without prior selection, revealing pathway shifts and novel species. It’s discovery-oriented and typically followed by targeted LC–MS/MS to confirm and quantify key changes.
Derivatization + HPLC/FLD
Fluorogenic tagging of sphingosine’s primary amine enables HPLC with fluorescence detection. Useful when MS access is limited; provides decent sensitivity at lower cost, but lower chemical specificity than MS and better suited to screening.
Enzyme activity assays (SphK readouts)
Fluorescent substrates such as NBD-sphingosine report sphingosine kinase conversion to NBD-S1P, enabling rapid, high-throughput assessment of pathway activity and inhibitor effects. Outputs relative activity, not absolute sphingosine levels.
Convenience kits / colorimetric workflows
Plate-based assays estimate sphingosine or S1P or related enzyme activity with minimal instrumentation. Fast and scalable for cell culture, but results are semi-quantitative and should be validated by mass spectrometry.
Use untargeted HRMS to discover what changes; confirm and quantify with targeted LC–MS/MS plus isotope dilution. Fluorescence and kit assays are practical for throughput and functional screening, not for definitive quantitation.
Partner with MetwareBio for Sphingosine Research
For studies centered on sphingosine, sphinganine, ceramides, and S1P, MetwareBio offers two complementary solutions:
- Quantitative Lipidomics : Absolute quantification with isotope-labeled internal standards and rigorous QA/QC—ideal for biomarker validation, pharmacodynamic tracking, and pathway analysis.
- Untargeted Metabolomics (Discovery): Broad, hypothesis-free profiling to reveal pathway shifts and novel lipid changes, followed by targeted verification where needed.
Our team helps you choose an approach that fits your samples and goals, so you can move from questions to actionable insights with confidence. Contact us to discuss your study needs.
FAQ: Common Questions About Sphingosine
Q: What is sphingosine (d18:1) vs. sphinganine (d18:0)?
A: Sphingosine is an 18-carbon amino alcohol with a 4,5-trans double bond (d18:1); sphinganine (dihydrosphingosine) is the saturated version (d18:0) and the immediate precursor in de novo sphingolipid synthesis.
Q: Is sphingosine the same as ceramide or sphingomyelin?
A: No. Ceramide = sphingoid base (e.g., sphingosine) + fatty acid (amide bond). Sphingomyelin = ceramide + phosphocholine headgroup. Sphingosine is a signaling backbone; ceramide/sphingomyelin are major membrane lipids.
Q: What is the sphingolipid rheostat?
A: The balance ceramide ↔ sphingosine ↔ S1P steers cell fate: ceramide/sphingosine tend to be pro-apoptotic, S1P pro-survival. Enzymes (ceramidase, SPHK1/2, S1P phosphatase/lyase) tune this balance.
Q: How do you measure sphingosine accurately?
A: Use targeted LC–MS/MS with isotope-labeled internal standards (e.g., d7-sphingosine) for specific, sensitive, absolute quantitation in plasma or tissues.
Q: Targeted sphingolipid profiling vs. global lipidomics—when to choose which?
A: Use targeted LC–MS/MS for defined metabolites and biomarker validation; use untargeted HRMS lipidomics for discovery, then confirm hits with targeted assays.
Q: Does sphingosine have antimicrobial activity?
A: Yes. Endogenous sphingosine on skin and airways disrupts bacterial membranes (e.g., S. aureus, P. aeruginosa). It’s a local defense lipid, not a systemic antibiotic.
Q: What do SPHK1 and SPHK2 do?
A: SPHK1 often promotes pro-survival/inflammatory S1P signaling; SPHK2 (including nuclear pools) can modulate gene expression and sometimes favors anti-inflammatory or growth-restraining effects—both are context-dependent.
Q: Are sphingolipids used in skincare?
A: Yes. Ceramide-rich formulations restore the skin barrier; phytosphingosine is used for antimicrobial/anti-inflammatory support in acne-prone skin.
In summary, sphingosine is far more than just a lipid molecule – it’s a central switch in cell signaling, a guardian in our skin and immune system, and a nexus point in diseases from eczema to cancer. Its “sphingolipid rheostat” function means tiny changes in sphingosine or its phosphate can tip the balance between cell death and survival. Because of this outsized influence, precise measurement and profiling of sphingosine and related sphingolipids are incredibly important in both research and clinical contexts.
References
- Wu Y, Liu Y, Gulbins E, Grassmé H. The Anti-Infectious Role of Sphingosine in Microbial Diseases. Cells. 2021;10(5):1105. doi:10.3390/cells10051105.
- Espaillat MP, Snider AJ. Ceramide and sphingosine-1-phosphate in cancer, two faces of the sphinx. Transl Cancer Res. 2015;4(5):484-499. doi:10.3978/j.issn.2218-676X.2015.10.01.
- Berkey R, Bendigeri D, Xiao S. Sphingolipids and plant defense/disease: the “death” connection and beyond. Front Plant Sci. 2012;3:68. doi:10.3389/fpls.2012.00068.
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139-150. doi:10.1038/nrm2329.
- Carter HE, et al. Biochemistry of the sphingolipides. III. Structure of sphingosine. J Biol Chem. 1947;170(1):285-295. doi:10.1016/S0021-9258(17)34955-4.
- Mallela SK, Merscher S, Fornoni A. Implications of Sphingolipid Metabolites in Kidney Diseases. International Journal of Molecular Sciences. 2022;23(8):4244. doi:10.3390/ijms23084244.
- Quinville BM, Deschenes NM, Ryckman AE, Walia JS. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. International Journal of Molecular Sciences. 2021;22(11):5793. doi:10.3390/ijms22115793.
- Song Z, Zhao Z, Liu X, et al. Sphingosine kinase 1 promotes M2 macrophage infiltration and enhances glioma cell migration via the JAK2/STAT3 pathway. Scientific Reports. 2025;15:4152. doi:10.1038/s41598-025-88328-2.
- Ji J, Wang J, Yang J, et al. The Intra-nuclear SphK2-S1P Axis Facilitates M1-to-M2 Shift of Microglia via Suppressing HDAC1-Mediated KLF4 Deacetylation. Frontiers in Immunology. 2019;10:1241. doi:10.3389/fimmu.2019.01241.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


