Trimethylamine N-oxide (TMAO): From Gut Microbiome Pathway to Cardiometabolic Risk
Trimethylamine N-oxide (TMAO) is a gut microbiome-derived metabolite formed when dietary nutrients like choline, carnitine, and betaine are metabolized into trimethylamine (TMA) and subsequently oxidized in the liver. In recent years, TMAO has gained prominence for its associations with cardiovascular disease (CVD), chronic kidney disease (CKD), type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). As a metabolite at the intersection of diet, gut microbiota, and host metabolism, TMAO is increasingly recognized as a critical biomarker in precision nutrition, cardiovascular risk stratification, and metabolic health.
This blog provides an in-depth overview of TMAO: what it is, how it’s produced, why it matters, its roles in chronic disease, strategies to lower it naturally, and how to test it accurately using targeted metabolomics platforms.
TMAO Explained: What It Is and Why It Matters
What is TMAO?
Trimethylamine N-oxide (TMAO) is a gut microbiome–derived metabolite formed through a two-step process. Dietary nutrients like choline, carnitine, and betaine—found in red meat, egg yolks, and fish—are first converted by gut bacteria into trimethylamine (TMA). TMA is then oxidized in the liver by the enzyme FMO3 to form TMAO. It is naturally abundant in marine animals as an osmoprotectant and is highly soluble, stable, and detectable in human plasma and urine.
Why TMAO Is Important
TMAO has emerged as a potential biomarker for cardiometabolic disease. Elevated circulating TMAO levels have been associated with increased risk of atherosclerosis, heart failure, chronic kidney disease (CKD), type 2 diabetes, and NAFLD. Experimental models suggest TMAO may alter cholesterol metabolism, impair vascular function, and promote inflammation. However, some researchers argue it may serve primarily as a marker of diet quality and gut dysbiosis. For instance, fish raises TMAO but lowers cardiovascular risk—indicating that context matters. TMAO is best interpreted as a microbiome-sensitive, diet-modulated risk signal that reflects complex host-microbe-nutrient interactions.
TMAO Discovery and Structure
How TMAO Was Discovered
TMAO was first identified in the 19th century as a byproduct of fish decomposition, responsible for the “fishy” odor when it breaks down into trimethylamine (TMA). In marine biology, it was recognized as a crucial osmolyte that helps deep-sea organisms stabilize proteins under extreme pressure. Its clinical relevance in humans emerged in 2011, when researchers at the Cleveland Clinic revealed that gut microbial metabolism of choline produces TMAO, and that elevated plasma TMAO predicts cardiovascular events like heart attack and stroke[1]. This pivotal study linked diet, the gut microbiome, and heart disease via a common metabolite.
Structural Characteristics of TMAO
Trimethylamine N-oxide (TMAO) is a small, water-soluble amine N-oxide with the molecular formula C₃H₉NO. It consists of a nitrogen atom bonded to three methyl groups and an oxygen atom. Unlike TMA, TMAO is odorless, chemically stable, and carries a polar N→O bond, which enhances its solubility and biological transport. In humans, TMAO is generated in the liver via the enzyme FMO3 and is excreted through the kidneys. Its stability and detectability make it an ideal target for LC-MS/MS–based metabolomics, often used in studies assessing diet–microbiome–host interactions.
TMAO Biosynthesis and Metabolic Pathway: Gut Microbiome & FMO3
How is TMAO synthesized in the body?
TMAO is produced through a host–microbiome–liver axis involving both microbial and enzymatic steps. Dietary compounds such as choline, carnitine, and betaine—found in red meat, eggs, and some supplements—are metabolized by gut microbiota into trimethylamine (TMA). This step depends on microbial enzymes like TMA lyases, with bacteria from genera such as Clostridia and Eggerthella contributing significantly [1]. After absorption into the portal vein, TMA reaches the liver, where it is oxidized by flavin-containing monooxygenase 3 (FMO3) into TMAO[2]. Genetic polymorphisms in the FMO3 gene can influence this conversion, and mutations can lead to trimethylaminuria (“fish odor syndrome”) [3]. The resulting TMAO is then released into the bloodstream and cleared primarily by the kidneys, with plasma levels strongly influenced by renal function.
TMAO’s role in host metabolic pathways
Beyond being a metabolic end-product, TMAO interacts with several physiological pathways. It influences cholesterol homeostasis by downregulating reverse cholesterol transport and modifying bile acid profiles [5]. TMAO has been shown to affect macrophage scavenger receptor expression, promoting foam cell formation—a hallmark of atherosclerosis [1][5]. Additionally, it intersects with phosphatidylcholine metabolism, impacting liver lipid handling. TMAO levels are also modulated by kidney clearance, and impaired renal function leads to TMAO accumulation, further amplifying cardiovascular risk [4]. Emerging evidence suggests that dietary fiber may reduce TMAO indirectly by enriching SCFA-producing bacteria, which compete with TMA producers and reduce microbial TMA output [6].
TMAO and Cardiovascular Disease: Atherosclerosis and Heart Risk
Trimethylamine N-oxide (TMAO) is a gut microbiome-derived metabolite (produced by hepatic FMO3 from dietary choline and carnitine in red meat) strongly linked to cardiovascular disease. It has emerged as a key factor connecting high red meat diets to increased cardiovascular risk via the gut microbiome, promoting atherosclerosis through effects on cholesterol metabolism and vascular inflammation [1,5].
In a multi-ethnic, ASCVD-free cohort (n=6,767), plasma TMAO showed a graded association with incident ASCVD across quintiles (HRs vs Q1: 1.02, 1.17, 1.23, 1.33; P-trend=0.01). Comparing the highest with the lowest quintile corresponded to a 33% higher risk of ASCVD (HR 1.33, 95% CI 1.02–1.74) after multivariable adjustment. Risk appeared larger among participants with lower baseline renal function, though interactions were not significant [15]. Mechanistically, TMAO can accelerate plaque formation by promoting foam cell formation and vascular inflammation. Interventions that block TMAO production in animal models significantly reduce plaque and thrombosis, underscoring TMAO’s potential causal role in CVD [5]. These findings highlight TMAO as a promising biomarker and potential therapeutic target for mitigating microbe-driven cardiovascular risk [4].
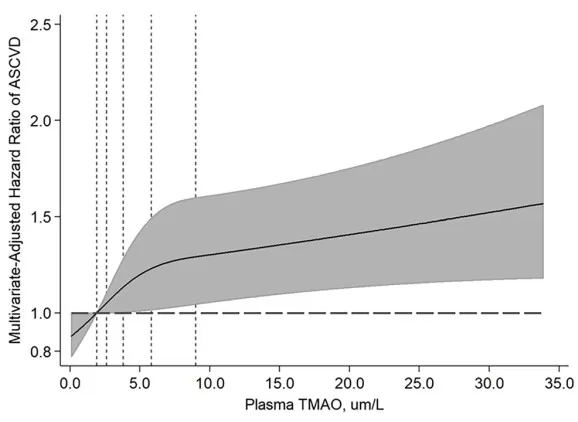
Multivariable-adjusted dose–response between plasma TMAO and incident ASCVD in a multi-ethnic cohort; hazard ratios increase across TMAO levels (shaded area: 95% CI).
Source: Adapted from Budoff MJ, de Oliveira Otto MC, Li XS, et al. “Trimethylamine-N-oxide (TMAO) and risk of incident cardiovascular events in the Multi-Ethnic Study of Atherosclerosis.” Scientific Reports. 2025;15:23362. DOI:10.1038/s41598-025-05903-3. © The Authors. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
TMAO and Kidney Disease: CKD and Renal Clearance
Chronic Kidney Disease (CKD) patients typically exhibit elevated TMAO levels due to reduced renal excretion (impaired kidney clearance). In healthy individuals, about 90% of TMAO is excreted by the kidneys; thus even moderate CKD allows TMAO to accumulate. Additionally, gut microbiome alterations in CKD may further increase TMAO generation. Far from a mere byproduct, TMAO can actively accelerate CKD progression by fueling renal inflammation and fibrosis, potentially creating a vicious cycle of TMAO accumulation and ongoing kidney damage [4,5].
In chronic kidney disease (CKD), reduced renal clearance leads to TMAO accumulation, and observational studies consistently report higher circulating TMAO with lower eGFR. Altered gut microbiota in CKD may further increase TMA generation. Thus, TMAO is both a marker of impaired renal function and a potential risk signal in CKD, warranting careful interpretation alongside kidney markers and diet. [4][5]
_1761534847_WNo_585d280.webp)
TMAO and TNF-α effects on renal fibroblasts. (A) Fibronectin release increases under TNF-α stimulation (1–50 ng/mL); co-exposure with TMAO maintains elevated profibrotic output. (B) LDH release remains low across conditions, indicating no overt cytotoxicity during the assay.
Source: Adapted from Lindgren D, Tamm M, Ragnarsdóttir B, et al. “TMAO enhances TNF-α-mediated fibrosis and release of inflammatory mediators from renal fibroblasts.” Scientific Reports. 2024;14:9070. DOI:10.1038/s41598-024-58084-w. © The Authors. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.*
TMAO and Type 2 Diabetes: Insulin Resistance and Inflammation
Type 2 Diabetes (T2D) patients tend to have higher TMAO levels, linking this gut microbial metabolite to insulin resistance and metabolic risk. TMAO has been implicated in chronic low-grade inflammation and altered glucose metabolism in diabetes, although causality remains under investigation. Animal studies also suggest that TMAO can impair glucose tolerance and exacerbate insulin resistance via inflammatory pathways [9].
For instance, a meta-analysis of over 15,000 participants found significantly higher circulating TMAO levels in individuals with T2D, with a dose-dependent relationship to diabetes prevalence[9]. Cross-sectional studies have likewise shown that TMAO correlates with higher fasting insulin and greater insulin resistance in humans [10]. However, prospective data are mixed – in one 2021 cohort, elevated TMAO did not significantly predict future diabetes onset despite its association with insulin resistance markers [10]. These findings suggest that while TMAO may contribute to metabolic disturbances (e.g. by promoting inflammation or altering bile acid signaling), it is likely not the sole cause of T2D. TMAO remains under active investigation as a potential biomarker or therapeutic target in diabetes.
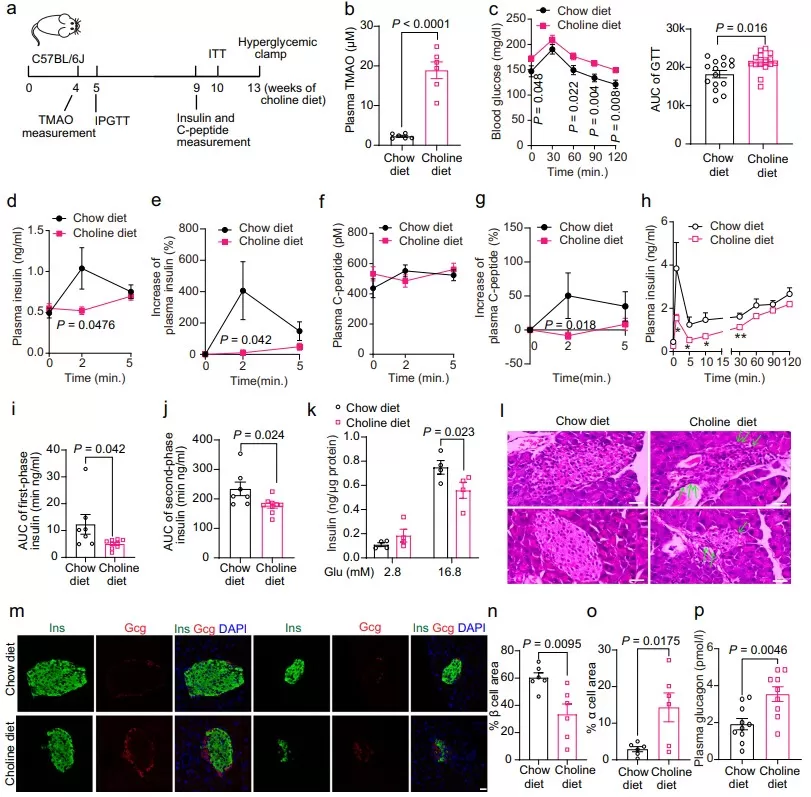
Choline diet elevates plasma TMAO and impairs β-cell function and glucose tolerance in mice. Panels show study design (a), higher plasma TMAO (b), worsened IPGTT (c), reduced insulin/C-peptide responses and GSIS under clamp (d–k), pancreatic histology (l), altered islet composition by immunostaining (m–o), and increased plasma glucagon (p), supporting a mechanistic link between TMAO and β-cell dysfunction.
Source: Adapted from Kong L, Zhao Q, Jiang X, et al. “Trimethylamine N-oxide impairs β-cell function and glucose tolerance.” Nature Communications. 2024;15:2526. DOI:10.1038/s41467-024-46829-0. © The Authors. Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No endorsement implied.
TMAO and Liver Disease: NAFLD and Metabolic Liver Dysfunction
Non-Alcoholic Fatty Liver Disease (NAFLD) – the buildup of fat in the liver unrelated to alcohol – has been linked to the gut microbial metabolite TMAO. NAFLD is highly prevalent (affecting ~25% of adults worldwide), and TMAO provides a tangible link between diet-driven gut microbiota changes and liver fat buildup with associated inflammation [6].
For example, a systematic meta-analysis (7 studies, >7,500 people) reported that NAFLD patients have significantly elevated circulating TMAO compared to healthy controls [11]. Experimental studies further demonstrate causality: dietary TMAO supplementation in animal models worsens hepatic steatosis and liver inflammation, activating stress pathways (e.g. the PERK endoplasmic reticulum stress response) and disrupting farnesoid X receptor (FXR) signaling [11]. Consistently, higher TMAO levels have been associated with more advanced liver fibrosis in NAFLD and progression to non-alcoholic steatohepatitis (NASH). These findings implicate TMAO as a driver in NAFLD pathogenesis via the gut microbiome–liver axis, and suggest that targeting TMAO production could be a novel strategy to mitigate NAFLD.
TMAO in Daily Life: Diet, Fish, and Fish Odor Syndrome
TMAO links dietary habits and the gut microbiome to health in surprisingly tangible ways. Its levels are mainly driven by food: diets rich in red meat and egg yolks increase TMAO via microbial metabolism of carnitine and choline, while fish contains pre-formed TMAO that spikes blood levels after meals. Interestingly, despite raising TMAO, fish consumption correlates with better cardiovascular outcomes, suggesting dietary context matters [4,5].
TMAO also explains phenomena like the "fishy smell" in spoiled seafood—caused by bacterial breakdown of TMAO into trimethylamine (TMA). In healthy individuals, FMO3 rapidly converts TMA to odorless TMAO. However, in trimethylaminuria (TMAU), a rare genetic disorder, deficient FMO3 leads to TMA accumulation and body odor. TMAU patients manage symptoms by limiting precursors (like red meat and eggs) and sometimes using antibiotics to suppress TMA-producing bacteria [3].
From a public-health perspective, high-fiber dietary patterns enrich microbiota that generate less TMA, whereas low-fiber, high-meat diets tend to increase TMAO production. Recent crossover data show that added fiber can blunt meat-associated TMAO surges in omnivores. [16]
Fish–TMAO Paradox: Why Seafood Can Raise TMAO Yet Support Heart Health
Seafood may acutely elevate circulating TMAO because it contains TMAO itself, but long-term fish consumption aligns with lower CVD risk in prospective evidence and is regularly recommended in heart-healthy dietary guidance. Prioritize whole-food fish (replacing red meat) within Mediterranean-style patterns rather than focusing on a single metabolite signal. [17]
How to Lower TMAO Levels Naturally
Lowering Trimethylamine N-oxide (TMAO) relies on modifying diet and supporting a healthy gut microbiome. Key strategies include:
- Eat more plant-based foods. Mediterranean and vegetarian diets are consistently linked to lower TMAO levels. In one RCT, a vegan diet reduced TMAO by 45% in just a week [12].
- Limit red meat and egg yolks. These are rich in carnitine and choline, key TMAO precursors. Replacing some meat with legumes or fish (despite its own TMAO) can lower microbial TMA generation [4,5].
- Increase dietary fiber. Fiber supports beneficial microbes that suppress TMA-producing strains. A recent crossover study found that fiber supplementation blunted post-meal TMAO surges in meat-eaters [13].
- Consider probiotics and prebiotics. While not yet definitive, certain strains (e.g., Bifidobacterium) may reduce TMA formation. Diverse, fiber-rich diets can help shape a TMAO-lowering microbiome.
- Avoid unnecessary supplements. Carnitine-containing fitness or energy products may increase TMAO levels.
Long-term, focusing on sustainable, gut-friendly dietary patterns offers the best strategy for lowering TMAO and improving cardiometabolic outcomes.
TMAO Testing in Research: Methods and Metabolomics Platforms
Trimethylamine N-oxide (TMAO) is frequently measured using LC-MS/MS in targeted metabolomics, offering high sensitivity and selectivity. It is commonly included in studies on cardiometabolic health, renal function, and microbiome-diet interactions [4].
TMAO is often profiled alongside its dietary precursors (choline, carnitine, betaine) and related lipid or bile acid metabolites. In metabolomics panels, it serves as a readout for gut microbial activity and FMO3 enzyme function. However, due to differences in chemical properties, some TMAO-related compounds (e.g. short-chain fatty acids, LPS) may require specialized methods such as HILIC or derivatization for optimal detection [14].
At Metware Bio, our TM Widely-Targeted Metabolomics platform enables high-throughput detection of many TMAO-associated metabolites, supporting your research in gut microbiome, nutritional metabolism, and cardiovascular disease.
FAQ: Scientific Questions About TMAO
Q1: Is TMAO good or bad?
A: Neither. TMAO is a diet- and microbiome-sensitive marker that correlates with cardiometabolic risk in several cohorts and may participate in pathways like cholesterol handling, inflammation, and bile acid signaling. But context matters: kidney function, habitual diet, and gut microbiota strongly influence levels. Treat TMAO as a risk signal, not a standalone diagnosis—interpret it alongside renal markers, diet pattern, and related metabolites (e.g., choline, carnitine, betaine).
Q2: Does eating fish raise TMAO—and should I worry?
A: Many fish contain pre-formed TMAO, so blood levels can spike transiently after a seafood meal. However, long-term fish consumption is consistently associated with lower CVD risk, likely due to omega-3s and the displacement of red/processed meat. If you’re measuring TMAO, avoid fish for 24–48 hours before sampling to get a baseline. Focus on the overall dietary pattern (more fiber, fewer processed meats) rather than a single metabolite, and don’t use TMAO alone as a reason to avoid fish.
Q3: What sample types are commonly used for TMAO detection?
A: Plasma and urine are most commonly used in clinical and metabolomics research. Plasma is generally preferred for consistency and cardiovascular relevance.
Q4: Can TMAO levels vary between individuals?
A: Yes. TMAO levels depend on factors like diet, gut microbiome composition, FMO3 genotype, and kidney function. There is wide inter-individual variability even with the same dietary intake [4].
Q5: Is TMAO routinely included in metabolomics studies?
A: Increasingly yes. It is frequently included in targeted metabolomics panels focused on cardiometabolic health, microbiome interactions, or renal clearance pathways [14].
Q6: Can antibiotics or probiotics affect TMAO levels?
A: Antibiotics can temporarily suppress TMA-producing bacteria, reducing TMAO levels. Some probiotics and fiber interventions may shift the microbiota to reduce TMAO formation, though more evidence is needed [6,13].
Q7: Is TMAO causally linked to disease, or just a marker?
A: Evidence suggests both. TMAO reflects diet-microbiome-host interactions and may also influence pathways like atherosclerosis, inflammation, and lipid metabolism [1,5,9].
References
1. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi:10.1038/nature09922.
2. Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi:10.1016/j.cmet.2012.12.011.
3. Al-Waiz M, Mitchell SC, Idle JR, Smith RL. The metabolism of 14C-trimethylamine and its N-oxide in man. Xenobiotica. 1987;17(5):551–558. doi:10.3109/00498258709043524.
4. Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi:10.1056/NEJMoa1109400.
5. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi:10.1038/nm.3145.
6. Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6(2):e02481-14. doi:10.1128/mBio.02481-14.
7. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7):e004947. doi:10.1161/JAHA.116.004947.
8. Gao J, Xu K, Liu H, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. doi:10.3389/fcimb.2018.00013.
9. Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular and metabolic risk biomarker: A systematic review and dose–response meta-analysis. Eur Heart J. 2019;40(34):2940–2949. doi:10.1093/eurheartj/ehz440.
10. Shan Z, Sun T, Huang H, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106(3):888–894. doi:10.3945/ajcn.117.156737.
11. Chen YM, Liu Y, Zhou RF, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi:10.1038/srep19076.
12. Rizzo G, Feraco A, Storz MA, et al. Vegan diets reduce plasma trimethylamine N-oxide (TMAO) concentrations: A systematic review and meta-analysis. Nutrients. 2023;15(7):1280. doi:10.3390/nu15071280.
13. Wilck N, Teltemann J, Müller DN. Dietary fiber reduces meat-associated TMAO spikes: Insights from the MEATMARK study. Eur J Clin Nutr. 2025. doi:10.1038/s41430-025-01636-8.
14. Wishart DS. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev. 2019;99(4):1819–1875. doi:10.1152/physrev.00035.2018.
15. Budoff MJ, de Oliveira Otto M, Li XS, et al. Trimethylamine-N-oxide and risk of incident cardiovascular events in the Multi-Ethnic Study of Atherosclerosis. Scientific Reports. 2025;15:23362. doi:10.1038/s41598-025-05903-3.
16. Haas M, Brandl B, Neuhaus K, Wudy S, Kleigrewe K, Hauner H, Skurk T. Effect of dietary fiber on trimethylamine-N-oxide production after beef consumption and on gut microbiota: MEATMARK – a randomized cross-over study. Eur J Clin Nutr. 2025;79:980-990. doi:10.1038/s41430-025-01636-8.
17. Wang Z, Tang WHW, O’Connell T, et al. Circulating trimethylamine N-oxide levels following fish or seafood consumption. Eur J Nutr. 2022;61(5):2357-2364. doi:10.1007/s00394-022-02803-4.
Read more
Malic Acid vs. Citric Acid: The Powerhouse Acids in Your Favorite Fruits
Fumaric Acid Unveiled: From Nature's Palette to Therapeutic Potential
Pyruvic Acid: A Key Player in Cellular Metabolism and Health
Lactic Acid: Key Roles in Human Metabolism, Diseases, and Health Implications
Cholic Acid: The Essential Bile Acid Impacting Digestion and Health
Kynurenine: The Hidden Metabolite Linking Immunity, Mental Health, and Disease Prevention
Understanding Glycine: Its Metabolism and Vital Role in Human Well-Being
Leucine: The Branched-Chain Amino Acid That Fuels Muscle Growth
Unveiling Ornithine: Beyond the Urea Cycle, A Multifaceted Player in Health
Tryptophan: Essential Amino Acid for Mood, Sleep, and More
Phenylalanine: Essential Roles, Metabolism, and Health Impacts
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


