What Is Sphingomyelin? Structure, Metabolism, and Its Role in Human Health
Sphingomyelin is the most abundant complex sphingolipid in animal cell membranes. It supports membrane architecture, organizes lipid rafts with cholesterol, and anchors signaling pathways that influence growth, differentiation, and apoptosis. Because it is a major lipid in the myelin sheath, sphingomyelin is tightly linked to brain development and neural transmission. This article explains what sphingomyelin is, details its structure, synthesis pathway and metabolism, highlights functions in human health and disease, summarizes dietary sources, and ends with practical next steps for targeted lipidomics.
What Is Sphingomyelin? Definition, Discovery, Structure—and Why It Matters
Sphingomyelin is an amphipathic membrane sphingophospholipid built from a sphingosine long-chain base plus a fatty acid (together forming ceramide) and a phosphorylcholine headgroup—i.e., “ceramide + phosphocholine.” In animal cells it is among the most abundant sphingolipids, organizing membranes and partnering with cholesterol to build ordered microdomains (“lipid rafts”). In nerves, sphingomyelin is enriched in the myelin sheath.
Sphingomyelin’s story begins in the 1880s, when J. L. W. Thudichum isolated “sphingolipids” from brain tissue and coined their Sphinx-inspired name to reflect their enigmatic chemistry. Its precise chemical structure—N-acyl-sphingosine-1-phosphorylcholine—was reported in 1927, cementing today’s definition and taxonomy.
At the molecular level, sphingomyelin structure comprises: (i) a C18-dominant sphingosine backbone; (ii) a saturated or monounsaturated C14–C26 fatty acyl chain linked at C2 to form ceramide; and (iii) a phosphorylcholine headgroup at C1. This quasi-cylindrical geometry packs tightly with cholesterol to form lipid rafts that cluster receptors and signaling proteins—one reason sphingomyelin is central to membrane order and receptor signaling.
Functionally, sphingomyelin is both structural scaffold and metabolic reservoir. Hydrolysis by sphingomyelinases generates ceramide, a stress-responsive second messenger; further metabolism yields sphingosine-1-phosphate (S1P), a pro-survival signal. The dynamic balance among sphingomyelin/ceramide/S1P underpins pathways relevant to brain development, cardiometabolic risk, and immune signaling—explaining why this lipid is so important across health and disease.
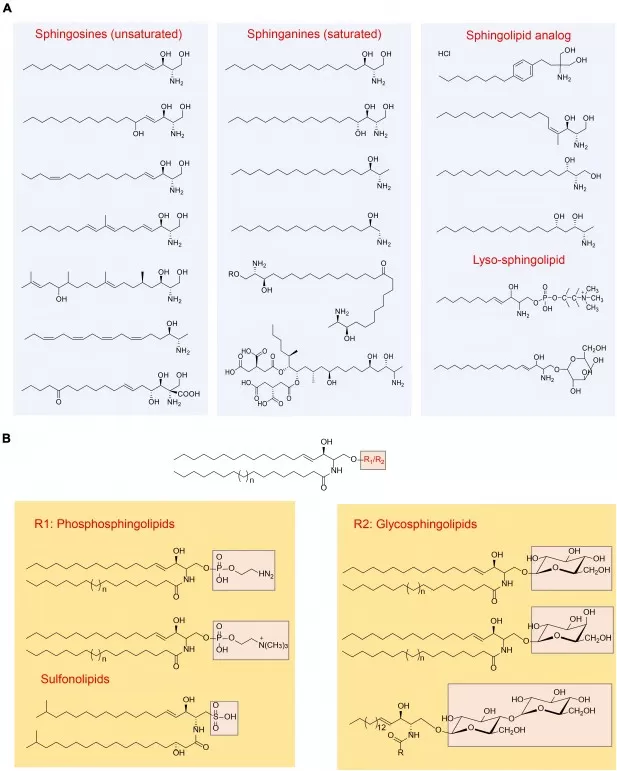
Figure 1. Key Sphingolipid Structures and Headgroups: Positioning Sphingomyelin within Phosphosphingolipids
Sphingomyelin Synthesis Pathway (SPT → CerS → SMS)
Sphingomyelin is built primarily via a de novo sphingomyelin synthesis pathway that spans the endoplasmic reticulum (ER) and the Golgi apparatus. In the ER, serine palmitoyltransferase (SPT) condenses serine with palmitoyl-CoA to form 3-ketosphinganine, which is reduced to dihydrosphingosine (sphinganine). Chain-length–specific ceramide synthases (CerS1–6) then N-acylate sphinganine with saturated/monounsaturated C14–C26 fatty acids to generate dihydroceramide, which DEGS1 desaturates to ceramide. Ceramide is ferried—largely by CERT—to the Golgi, where sphingomyelin synthase (SMS1/2) transfers phosphocholine from phosphatidylcholine to ceramide, yielding sphingomyelin and diacylglycerol (DAG). This ER→Golgi pipeline lets cells fine-tune species composition by combining distinct long-chain bases and acyl chains. In parallel, dietary sphingomyelin (notably from eggs, milk/dairy, and soy) is hydrolyzed in the gut by alkaline sphingomyelinase/ENPP7 and neutral sphingomyelinases to ceramide and sphingosine, which are absorbed and reused for complex sphingolipid synthesis. Although endogenous production dominates, typical Western diets (~200–400 mg/day sphingolipids) can modestly shape circulating sphingomyelin profiles—especially relevant in early life and nutrition studies.
Metabolic Dynamics: Sphingomyelin Degradation and Signal Switching
Once in membranes, sphingomyelin participates in a dynamic sphingomyelin–ceramide–S1P axis that integrates stress and growth signals. Sphingomyelinases (acid, neutral, alkaline) hydrolyze sphingomyelin to ceramide in response to TNF-α, IL-1, UV/radiation, and oxidative stress, often within cholesterol-rich lipid rafts. Ceramide can activate PKCζ, PP2A (CAPP), and other effectors, promoting growth arrest or apoptosis; it is further deacylated by ceramidases to sphingosine, then phosphorylated by sphingosine kinases (SphK1/2) to sphingosine-1-phosphate (S1P). S1P signals through GPCRs to drive survival, proliferation, and migration, and can be degraded by S1P lyase for irreversible flux. The relative levels of ceramide vs. S1P—the ceramide–S1P rheostat—help determine cell fate and are implicated in neurodegeneration, Niemann–Pick disease (ASM deficiency), insulin resistance, and atherosclerosis. This continuous remodeling also feeds salvage routes, replenishing ceramide for resynthesis of sphingomyelin in the Golgi, linking metabolism to signaling outcomes.
Sphingomyelin and Neurological Health (Myelin Sheath, Lipid Rafts, Neurodegeneration)
As a core membrane lipid, sphingomyelin supports the myelin sheath that insulates axons and organizes lipid rafts that cluster receptors on neurons and glia—foundational to fast conduction, synaptic signaling, and neuroimmune balance. During development, adequate sphingomyelin helps build and refine myelin; in adulthood, shifts in sphingomyelin metabolism toward ceramide (and away from S1P) can amplify pro-apoptotic signaling, oligodendrocyte stress, and demyelination. These dynamics help explain why sphingomyelin function is relevant to multiple conditions—from myelin injury (e.g., MS-like processes) to mood circuitry where acid sphingomyelinase activity and ceramide levels modulate stress pathways. In short, maintaining a healthy sphingomyelin–ceramide–S1P balance is integral to neurological resilience.
As a concrete illustration of this principle, a recent high-impact study in Parkinson’s disease reported that ten sphingomyelin species were significantly increased in the amygdala (but not in visual cortex), with total sphingomyelin rising alongside disease duration. The authors also observed ABCA5 upregulation correlating with sphingomyelin—suggesting a compensatory role in clearing excess lysosomal SM—and found selected SM species elevated in plasma, pointing to translational biomarker potential. Taken together, the case supports a broader idea: dysregulated sphingomyelin metabolism can contribute to neuronal stress and proteinopathy, reinforcing why species-resolved lipidomics of sphingomyelin is valuable for understanding—and potentially monitoring—neurodegeneration.
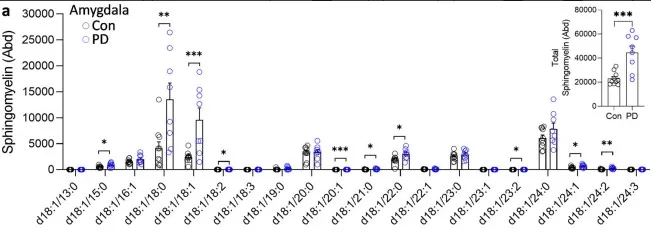
Figure 2. LC-MS analysis of sphingomyelin species in sporadic PD brain tissues
Sphingomyelin Metabolism in Cardiometabolic Disease: A Double-Edged Sword
In circulation and metabolic health, sphingomyelin function is inherently two-sided: as a major phospholipid on LDL it partners with cholesterol—affecting particle aggregation, arterial retention, and thus atherogenic potential (sphingomyelin and cholesterol)—while flux through the sphingomyelin–ceramide–S1P axis ties sphingomyelin metabolism to inflammation and insulin signaling (sphingomyelin ceramide S1P). This macro picture is mirrored by human evidence: in the Cardiovascular Health Study (JAMA Network Open, 2023), each 1-SD higher SM 16:0 associated with ~37% greater risk of sudden cardiac death, independent of traditional risk factors; complementary population data (Clinical Chemistry, 2021) showed SM 16:0/Cer 16:0 linked to higher all-cause mortality, whereas longer-chain species (e.g., SM 22:0/24:0) were neutral or inversely related—underscoring that not all “sphingomyelin” behaves the same. Although endogenous synthesis dominates, dietary sphingomyelin (sphingomyelin in diet) may modestly modulate intestinal lipid handling and lipoprotein profiles, supporting the idea that rebalancing species composition and pathway flux—rather than simply raising total SM—offers more promise for managing sphingomyelin diseases within the cardiometabolic spectrum.
Sphingomyelin in Cancer and Immunity (Tumor Immune Evasion, NK Cells, Sphingomyelinase Inhibition)
Dysregulated sphingomyelin metabolism is tightly linked to tumorigenesis and immune control. Under homeostasis, pro-apoptotic ceramide is generated from sphingomyelin by sphingomyelinases in response to death signals, helping clear abnormal cells. Many cancers rewire this axis—often accumulating sphingomyelin while reducing ceramide—via elevated sphingomyelin synthase and/or reduced sphingomyelinase activity, creating a “high-SM/low-ceramide” state that blunts apoptosis and favors tumor immune evasion. In infection and host defense, pathogens exploit sphingomyelin-rich lipid rafts (e.g., viral docking) or secrete sphingomyelinases to damage membranes, whereas lymphocyte activation depends on SM-enriched microdomains to assemble receptor signaling platforms. As direct, human evidence supporting this view, a 2023 Nature Immunology study in liver cancer showed intratumoral NK cells have depleted membrane sphingomyelin; inhibiting sphingomyelinase restored surface SM, rebuilt protrusions and lytic synapses, revived cytotoxicity, and synergized with checkpoint blockade—summarized by a Nature commentary as a metabolically targetable “immune checkpoint.” Together, these findings place the sphingomyelin–ceramide–S1P flux at the heart of oncology and immunology strategies, highlighting sphingomyelinase inhibition and pathway rebalancing as viable complements to modern immunotherapy.
_1755669369_WNo_589d372.webp)
Figure 3. Reduced Membrane Sphingomyelin in Intratumoral NK Cells
Acid Sphingomyelinase Deficiency (Niemann–Pick A/B): Lysosomal Accumulation and Clinical Translation
Defects in sphingomyelin metabolism can culminate in lysosomal storage pathology, epitomized by acid sphingomyelinase deficiency (ASMD; Niemann–Pick A/B). When ASM activity is absent or reduced, sphingomyelin and cholesterol accumulate—especially in macrophages—driving progressive splenomegaly/hepatomegaly, interstitial lung disease, and, in severe type A, early neurovisceral involvement; type B typically presents later with limited CNS features. This clinicopathologic spectrum underscores a simple principle: without efficient sphingomyelin catabolism, cellular “waste” builds up and multiple organs suffer. Importantly, the pathway is actionable—as shown in adults with ASMD in the phase 2/3 ASCEND randomized trial, where olipudase alfa (recombinant ASM) produced significant improvements in DLCO and marked reductions in spleen and liver volumes at 52 weeks versus placebo, with benefits sustained beyond year 2; these outcomes have informed regulatory approvals for pediatric and adult use. In short, ASMD is both a cautionary tale about disrupted sphingomyelin metabolism and a proof-of-concept that correcting the defective step can deliver tangible clinical benefit.
_1755669460_WNo_975d359.webp)
Figure 4. Spleen endpoints over time in ASCEND
Sphingomyelin in Plants: A Different Stage for Related Sphingolipids
Higher plants do not contain classical sphingomyelin (the phosphocholine-headed sphingolipid found in animals). Instead, plant membranes are rich in sphingomyelin-related sphingolipids—primarily glycosphingolipids, inositol-phosphorylceramides, and diverse ceramides, often with very-long-chain fatty acids. These lipids build and stabilize the plasma membrane and endomembrane systems, help organize membrane microdomains, and support normal growth and tissue formation. Beyond structure, plant sphingolipids act as signals in environmental responses: phyto-S1P and related metabolites link to stomatal regulation and drought response, while sphingolipid turnover participates in pattern-triggered immunity, where rapid changes in ceramide and complex sphingolipids can promote defensive gene expression and controlled cell death at infection sites. Certain pathogens and toxins can disrupt sphingolipid synthesis, weakening these defenses, whereas maintaining balanced sphingolipid metabolism enhances membrane integrity and stress resilience. In short, although plants lack sphingomyelin itself, a network of sphingomyelin-related sphingolipids fulfills comparable roles in membrane structure and signal transduction, making them important readouts for plant lipidomics and stress-biology studies.
Sphingomyelin in Daily Life
In everyday terms, sphingomyelin helps keep cell membranes organized (lipid rafts) and supports the myelin sheath in the nervous system—so its role is structural and signaling, not a quick “boost.” Because the body synthesizes sphingomyelin, true deficiency is uncommon (outside rare genetic disorders); for most people, diet fine-tunes the profile rather than “fixes a lack.” If you’re asking “what is sphingomyelin good for?”, studies link dietary SM to modest effects on cholesterol absorption and gut barrier support, especially relevant in early life. As for sphingomyelin in food, the richest everyday sources are egg yolks, milk and dairy, and soy, while fruits and most vegetables contain very little. Breast milk naturally provides SM, and some infant formulas are fortified. For adults, a balanced diet is usually sufficient; dietary sphingomyelin supplements and skin-oriented products exist, but evidence is still emerging.
From supporting membrane architecture to orchestrating cell signaling—and from neural transmission to metabolic homeostasis—sphingomyelin plays essential roles across biology. A deeper understanding of sphingomyelin and its metabolic network can illuminate mechanisms underlying major diseases and suggest new avenues for prevention and intervention. Indeed, metabolic modulation of the sphingomyelin pathway is emerging as a research focus in areas such as stroke and diabetic complications. For researchers and clinicians alike, accurate quantification of sphingomyelin and related sphingolipids is foundational to study design, biomarker discovery, and translational work.
Frequently Asked Questions
Q1. What is sphingomyelin used for in the body?
It stabilizes membranes, forms lipid rafts with cholesterol, supports myelin sheath structure, and serves as a reservoir for bioactive lipids (ceramide, S1P) that regulate cell fate.
Q2. Is sphingomyelin good or bad for health?
It’s essential for normal physiology. Imbalance—too much in certain lipoproteins or too much ceramide signaling—can contribute to atherosclerosis, insulin resistance, or neurodegeneration. Health is about maintaining balanced metabolism.
Q3. How is sphingomyelin made? (Sphingomyelin synthesis pathway)
SPT builds the long-chain base → CerS form ceramide → sphingomyelin synthase adds a phosphorylcholine headgroup in the Golgi. Degradation by sphingomyelinase regenerates ceramide.
Q4. Which foods contain sphingomyelin? (Sphingomyelin in food)
Highest levels appear in egg yolks, milk/dairy, and soy; levels are low in fruits and most vegetables. Human breast milk is naturally rich for infant needs.
Q5. Is sphingomyelin linked to heart disease or diabetes?
Elevated LDL-sphingomyelin is associated with atherogenicity. Ceramide accumulation impairs insulin signaling, connecting sphingolipid metabolism to type 2 diabetes risk.
Advance Your Sphingomyelin Research with MetwareBio Lipidomics Services
MetwareBio is a leading provider of proteomics, metabolomics, multi-omics and spatial metabolomics solutions, with an advanced quantitative lipidomics platform capable of profiling thousands of lipid species, including sphingomyelin.Using high-sensitivity mass spectrometry and standardized workflows, MetwareBio delivers precise, reliable measurements to help map sphingomyelin dynamics across biological processes and disease states. Backed by extensive project experience and a specialized technical team, we offer end-to-end support—from sample preparation and measurement to statistical analysis and interpretation. Whether your focus is neural sphingomyelin changes, cardiovascular lipid biomarkers, or sphingolipid composition in plant samples, MetwareBio will help you obtain robust datasets efficiently and accelerate your research. Partner with MetwareBio to accelerate sphingomyelin research and turn data into decision-ready results.
Read more
- What is Phospholipid? Structure, Functions, and Applications
- Unveiling Ornithine: Beyond the Urea Cycle, A Multifaceted Player in Health
- Malic Acid vs. Citric Acid: The Powerhouse Acids in Your Favorite Fruits
- Fumaric Acid Unveiled: From Nature's Palette to Therapeutic Potential
- Pyruvic Acid: A Key Player in Cellular Metabolism and Health
- Lactic Acid: Key Roles in Human Metabolism, Diseases, and Health Implications
- Cholic Acid: The Essential Bile Acid Impacting Digestion and Health
- Kynurenine: The Hidden Metabolite Linking Immunity, Mental Health, and Disease Prevention
Reference
- Bai X, Ya R, Tang X, Cai M. Role and interaction of bacterial sphingolipids in human health. Front Microbiol. 2023;14:1289819. Published 2023 Oct 23. doi:10.3389/fmicb.2023.1289819
- Fu Y, Pickford R, Galper J, et al. A protective role of ABCA5 in response to elevated sphingomyelin levels in Parkinson's disease. NPJ Parkinsons Dis. 2024;10(1):20. Published 2024 Jan 11. doi:10.1038/s41531-024-00632-2
- Bockus LB, Jensen PN, Fretts AM, et al. Plasma Ceramides and Sphingomyelins and Sudden Cardiac Death in the Cardiovascular Health Study. JAMA Netw Open. 2023;6(11):e2343854. Published 2023 Nov 1. doi:10.1001/jamanetworkopen.2023.43854
- Fretts AM, Jensen PN, Hoofnagle AN, et al. Circulating Ceramides and Sphingomyelins and Risk of Mortality: The Cardiovascular Health Study. Clin Chem. 2021;67(12):1650-1659. doi:10.1093/clinchem/hvab182
- Zheng X, Hou Z, Qian Y, et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat Immunol. 2023;24(5):802-813. doi:10.1038/s41590-023-01462-9
- Wasserstein M, Lachmann R, Hollak C, et al. A randomized, placebo-controlled clinical trial evaluating olipudase alfa enzyme replacement therapy for chronic acid sphingomyelinase deficiency (ASMD) in adults: One-year results. Genet Med. 2022;24(7):1425-1436. doi:10.1016/j.gim.2022.03.021
- Wasserstein MP, Lachmann R, Hollak C, et al. Continued improvement in disease manifestations of acid sphingomyelinase deficiency for adults with up to 2 years of olipudase alfa treatment: open-label extension of the ASCEND trial. Orphanet J Rare Dis. 2023;18(1):378. Published 2023 Dec 2. doi:10.1186/s13023-023-02983-0
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


